Hcv Rna Viral Load Normal Range

Summary From sld 15 For Hepatitis C

Treatment Of Hcv Infection By Targeting Microrna Nejm

Modeling Of Patient Virus Titers Suggests That Availability Of A Vaccine Could Reduce Hepatitis C Virus Transmission Among Injecting Drug Users Science Translational Medicine

Comparison Between Patients With Or Without Intrahepatic Hepatitis C Download Table

Quantitation Of Hcv Rna In Liver Of Patients With Chronic Hepatitis C

Asociacion Entre Factores Angiogenicos Solubles Y Marcadores De Progresion De La Enfermedad En Pacientes Con Hepatitis Cronica C
A viral load of less than 615 IU/mL means there’s no detectable HCV, or it’s too low to detect.

Hcv rna viral load normal range. When you have a viral load test, the results are typically reported in international units per milliliter (IU/mL) and copies per milliliter. Doctors usually consider a viral load over 800,000 IU/ml as high. Low if less than 800,000 IU/mL;.
At the end of. An "Undetected" result indicates that the HCV is absent in the patient's serum specimen. If a qualitative RNA test is positive (detected), then it is confirmed that the patient has chronic hepatitis C.
If your results are:. The virus is detected, but the amount can’t be measured. A low viral load would be less than 800,000 IU/ml.
If viral load testing does not find any HCV, your viral load is called “undetectable.”. If a quantitative test indicates undetectable viral particle levels, but the. High if greater than 800,000 IU/mL;.
For each patient, the result can be described as either a "high" viral load, which is usually >800,000 IU/L, or a "low" viral load, which is usually <800,000 IU/L. Your odds that treatment will make all or most of your HCV go away are better than with a high viral load. This assay has a result range of 15 to 100,000,000 IU/mL (1.18 log to 8.00 log IU/mL) for quantification of hepatitis C virus (HCV) RNA in serum.
The viral load results from the quantitative PCR test can range from 15 to 100,000,000 IU/L. Tend to detect viral load amounts as low as copies/mL, slightly older tests can't detect amounts below 50 copies/mL -- and there are still some tests in use. The qualitative HCV RNA tests will report whether the hepatitis C virus is present in the bloodstream or not.
It's not uncommon to have a viral load in the millions. The result is reported as either "detected" or "not detected." Explanation of test results:. During treatment, a falling viral load is an indication that treatment is succeeding.
This is a count below 800,000 IU/mL. § If the person tested is suspected of having HCV exposure within the past 6 months, or has clinical evidence of HCV disease, or if there is concern regarding the handling or storage of the test specimen. The quantitative HCV RNA test is checked before a patient starts treatment.
† It is recommended before initiating antiviral therapy to retest for HCV RNA in a subsequent blood sample to confirm HCV RNA positivity.

Hepatitis C Testing Hepatitis C Treatment

Hepatitis C Virus And Non Hodgkin S Lymphomas A Minireview Sciencedirect
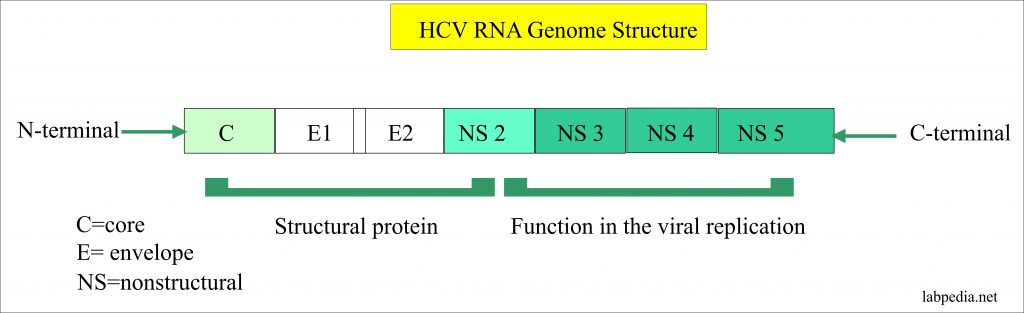
Hepatitis C Virus Part 1 Hepatitis C Virus Hcv Profile Labpedia Net

Serum And Liver Hcv Rna Levels In Patients With Chronic Hepatitis C Correlation With Clinical And Histological Features Gut
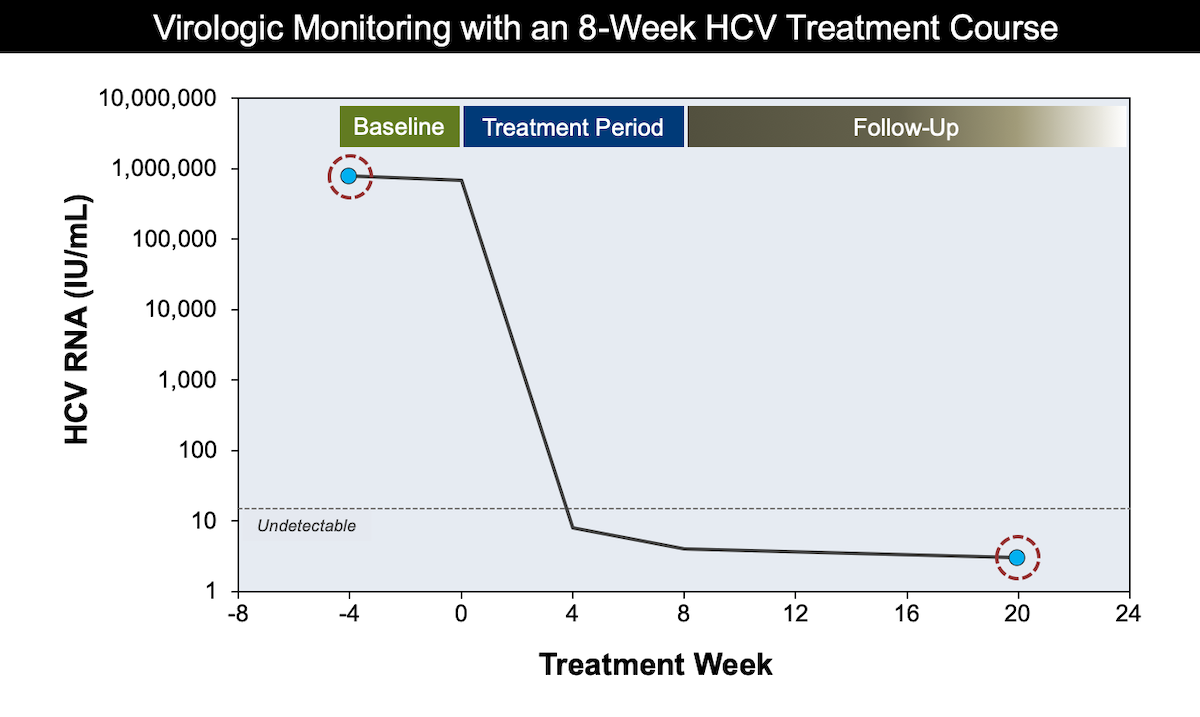
Monitoring During And After Hcv Treatment Core Concepts
Q Tbn 3aand9gcrj4qrha316ka4rodbkcgfu1bqrk1siqizw12e66mmvr8tbrdrm Usqp Cau
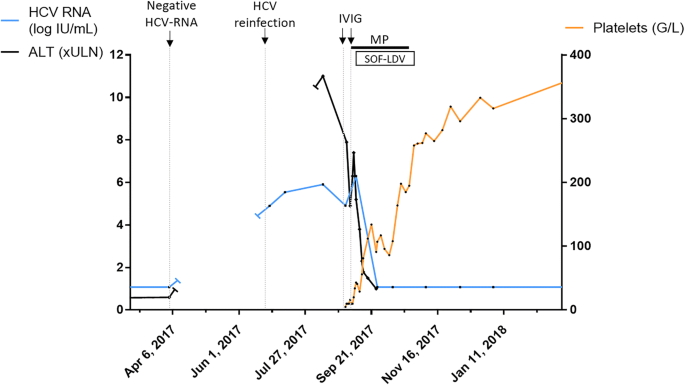
Early Sofosbuvir Ledipasvir Treatment For Acute Hcv Infection Induced Severe Immune Thrombocytopenia A Case Report Bmc Infectious Diseases Full Text
Correlation Of Viral Loads With Hcv Genotypes Higher Levels Of Virus Were Revealed Among Blood Donors Infected With 6a Strains
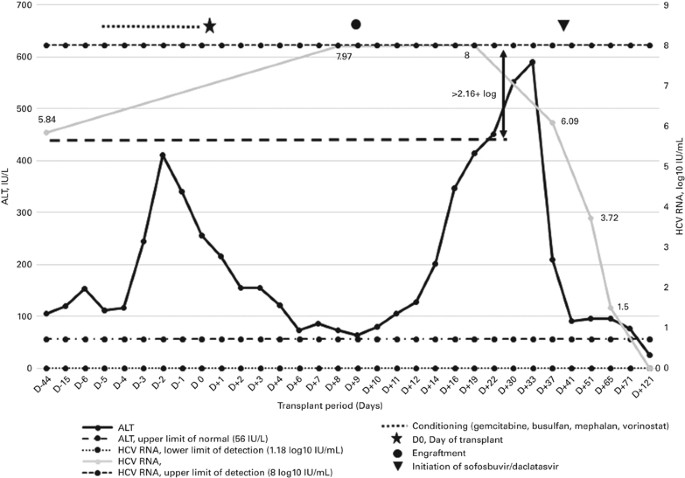
Severe Hepatitis C Reactivation As An Early Complication Of Hematopoietic Cell Transplantation Bone Marrow Transplantation
Q Tbn 3aand9gct7lehfgx2xlkxzmjkk33wcdkpdyijhyl6bn4kw7ne Usqp Cau

Treatment Of Acute Hepatitis C With Interferon Alfa 2b Nejm

Table 1 From Evaluation Of Performances Of Versant Hcv Rna 1 0 Assay Kpcr And Roche Cobas Ampliprep Cobas Taqman Hcv Test V2 0 At Low Level Viremia Semantic Scholar

Pdf Performance Characteristics Of A Real Time Rt Pcr Assay For Quantification Of Hepatitis C Virus Rna In Patients With Genotype 1 And 2 Infections
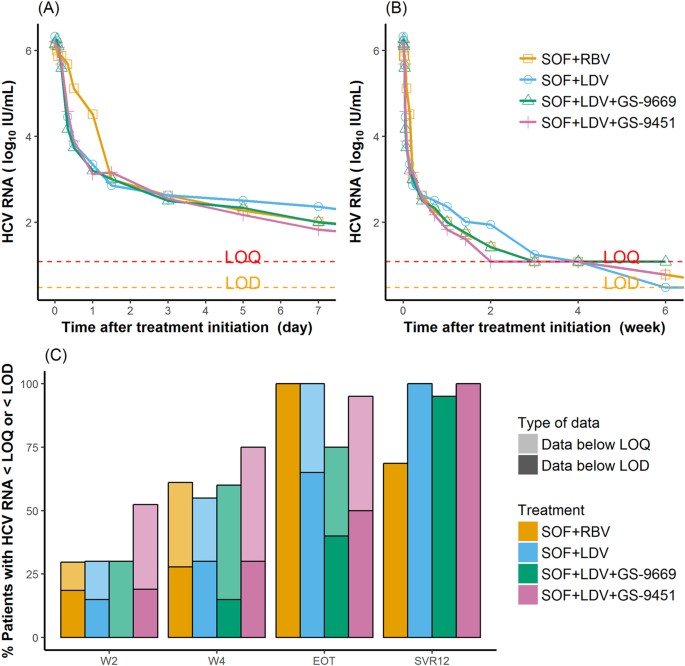
The Paradox Of Highly Effective Sofosbuvir Based Combination Therapy Despite Slow Viral Decline Can We Still Rely On Viral Kinetics Scientific Reports
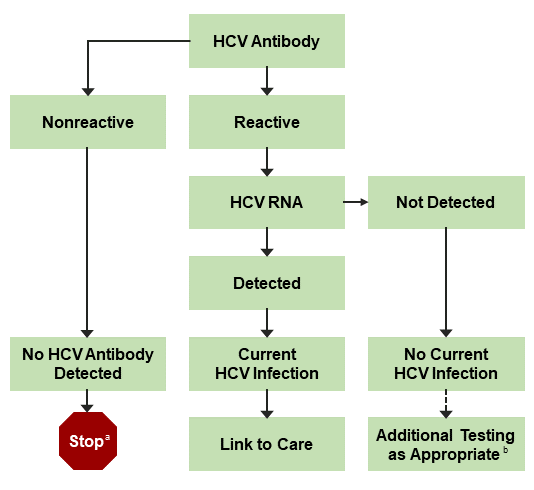
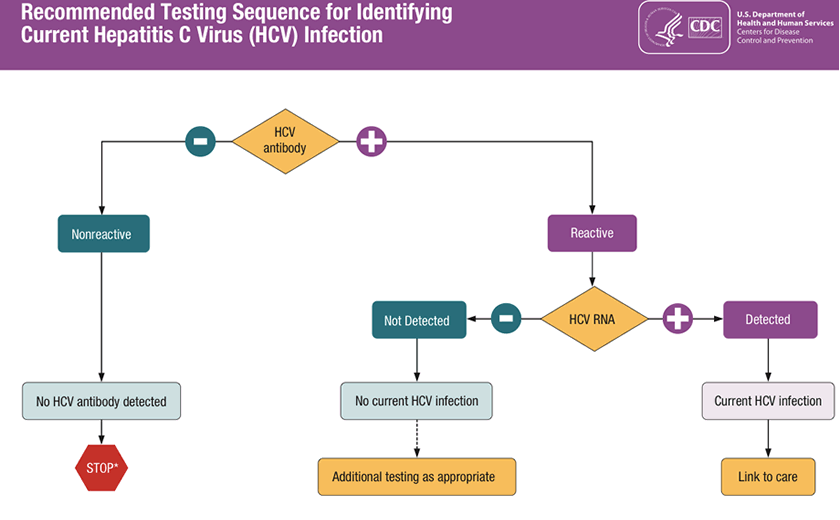
Hcv Testing And Linkage To Care Hcv Guidance

Rapid Decline Of Viral Rna In Hepatitis C Patients Treated With Vx 950 A Phase Ib Placebo Controlled Randomized Study Gastroenterology

Hcv Rna Viral Load Log Iu Ml Download Table

Hepatitis C Testing Hepatitis C Treatment
A Higher Correlation Of Hcv Core Antigen With Cd4 T Cell Counts Compared With Hcv Rna In Hcv Hiv 1 Coinfected Patients

Utility Of Routine Real Time Quantitative Pcr Monitoring Of Hcv Infection In Haemodialysis Patients Datta S Goel N Wattal C Indian J Med Microbiol
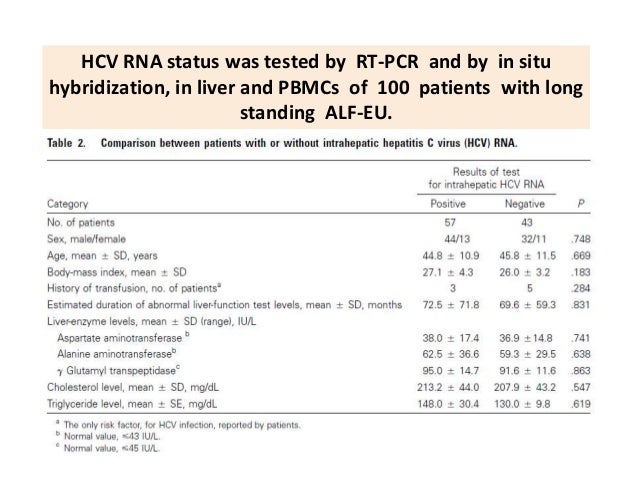
Occult Hcv Infection The Updated Knowledge
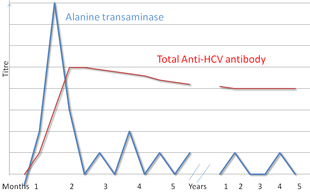
Hepatitis C Wikipedia

Distribution Pattern Of Hcv Genotypes Its Association With Viral Load

Hepatitis C Virus Rna Quantitation In Venous And Capillary Small Volume Whole Blood Samples Journal Of Clinical Microbiology
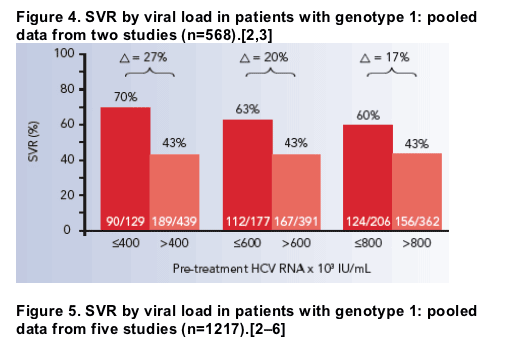
400 000 Iu Ml Is New Cut Off For Low Vs High Viral Load In Hcv
Variation Of Transaminases Hcv Rna Levels And Th1 Th2 Cytokine Production During The Post Partum Period In Pregnant Women With Chronic Hepatitis C

Hepatitis C Virus Core Antigen A Simplified Treatment Monitoring Tool Including For Post Treatment Relapse Sciencedirect
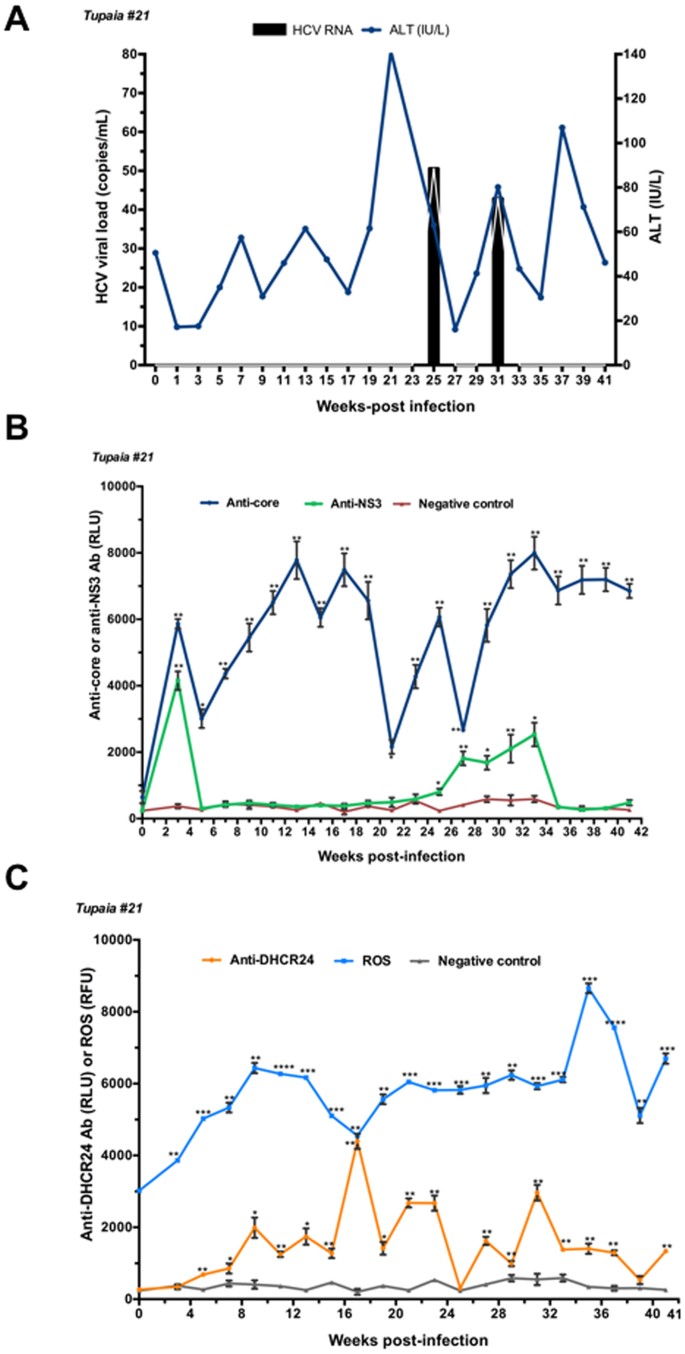
Oxidative Stress And Immune Responses During Hepatitis C Virus Infection In Tupaia Belangeri Scientific Reports

Cell Culture Grown Hepatitis C Virus Is Infectious In Vivo And Can Be Recultured In Vitro Pnas

Range Of Hcv Rna Titers According To Histologic Activity Index Hai By Download Table
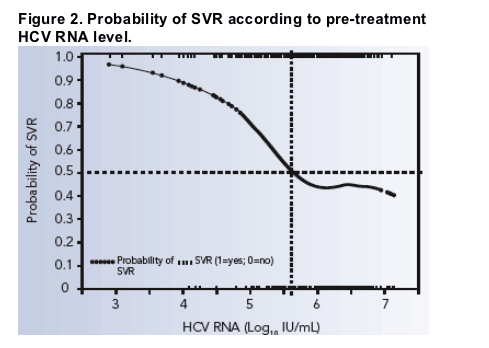
400 000 Iu Ml Is New Cut Off For Low Vs High Viral Load In Hcv

Hepatitis C Viral Load Genotype And Increased Risk Of Developing End Stage Renal Disease Reveal Hcv Study Lai 17 Hepatology Wiley Online Library

What Is Hepatitis C Viral Load

Occult Hepatitis C Virus Infection A Review
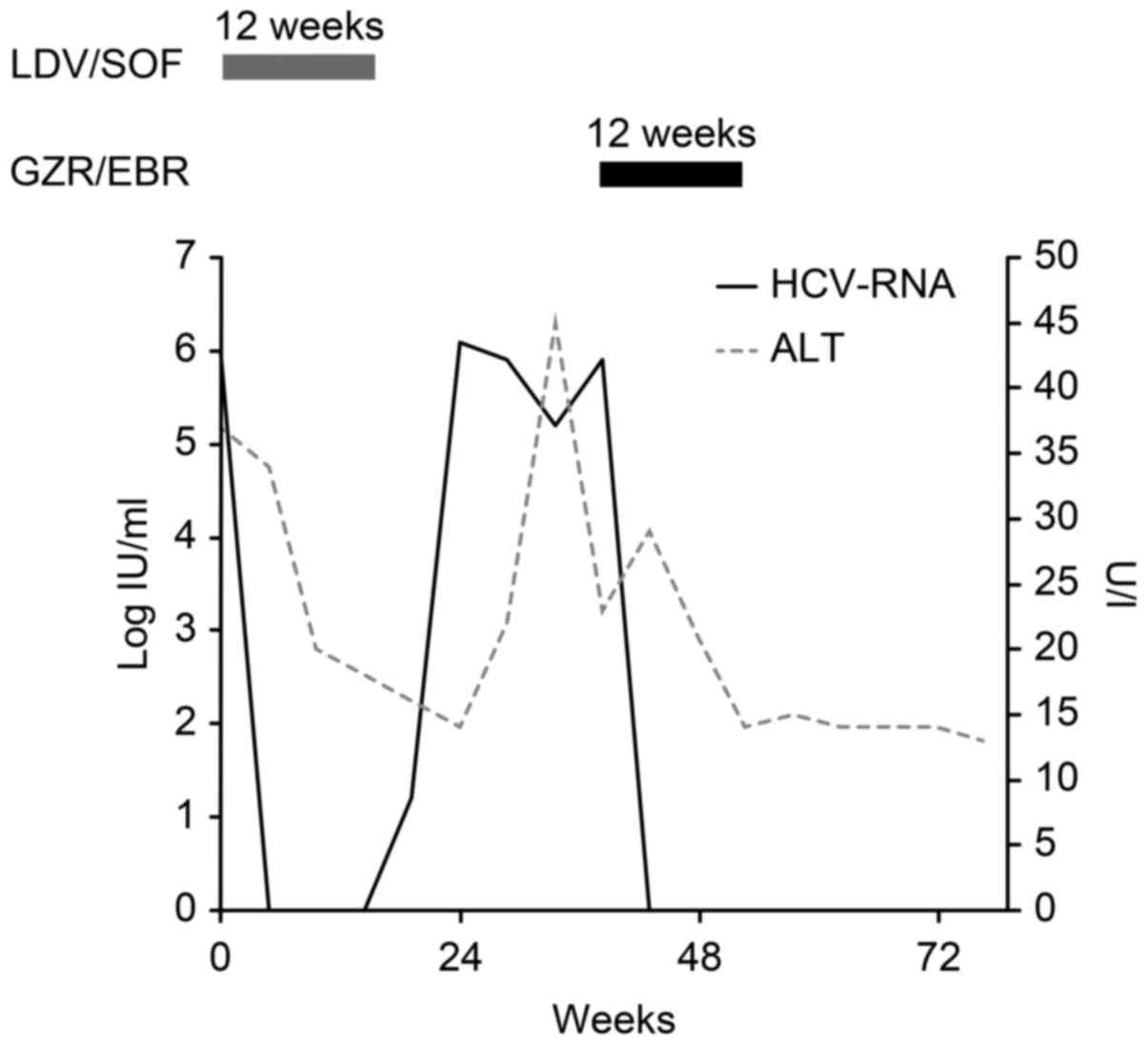
Grazoprevir Elbasvir Treatment For The Relapse Of Hcv Genotype 1b Infection After Ledipasvir Sofosbuvir A Case Report
Case Of The Month False Positive Hiv Viral Loads National Clinician Consultation Center

Positive Test Results Obtained By The Three Quantitative Hcv Rna Assays Download Table

Serum And Liver Hcv Rna Levels In Patients With Chronic Hepatitis C Correlation With Clinical And Histological Features Gut

Correlations Of Viral Load Toward Hcv Core Antigen And Aminotransferase In Hepatitis C Virus Genotype 1 Infection Scialert Responsive Version
Untitled Document

Hepatitis C Virus Core Antigen Assay Can We Think Beyond Convention In Resource Limited Settings
Q Tbn 3aand9gcs7dwjlfhd3 U4n469qbcmudgsgm 6cv6nwtiyycealosd1xsyb Usqp Cau
Www Hepatitisc Uw Edu Pdf Treatment Infection Monitoring Core Concept All
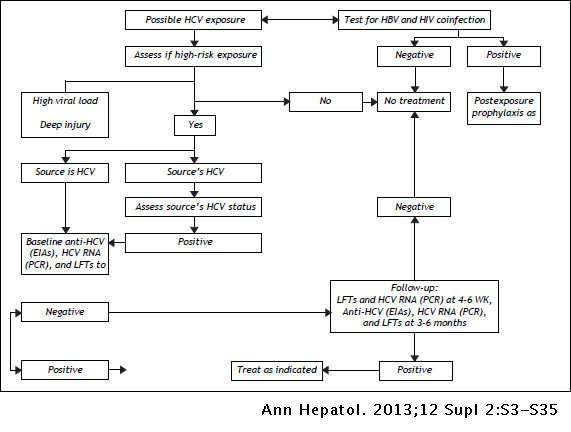
An Update On The Management Of Hepatitis C Guidelines For Protease Inhibitor Based Triple Therapy From The Latin American Association For The Study Of The Liver Annals Of Hepatology
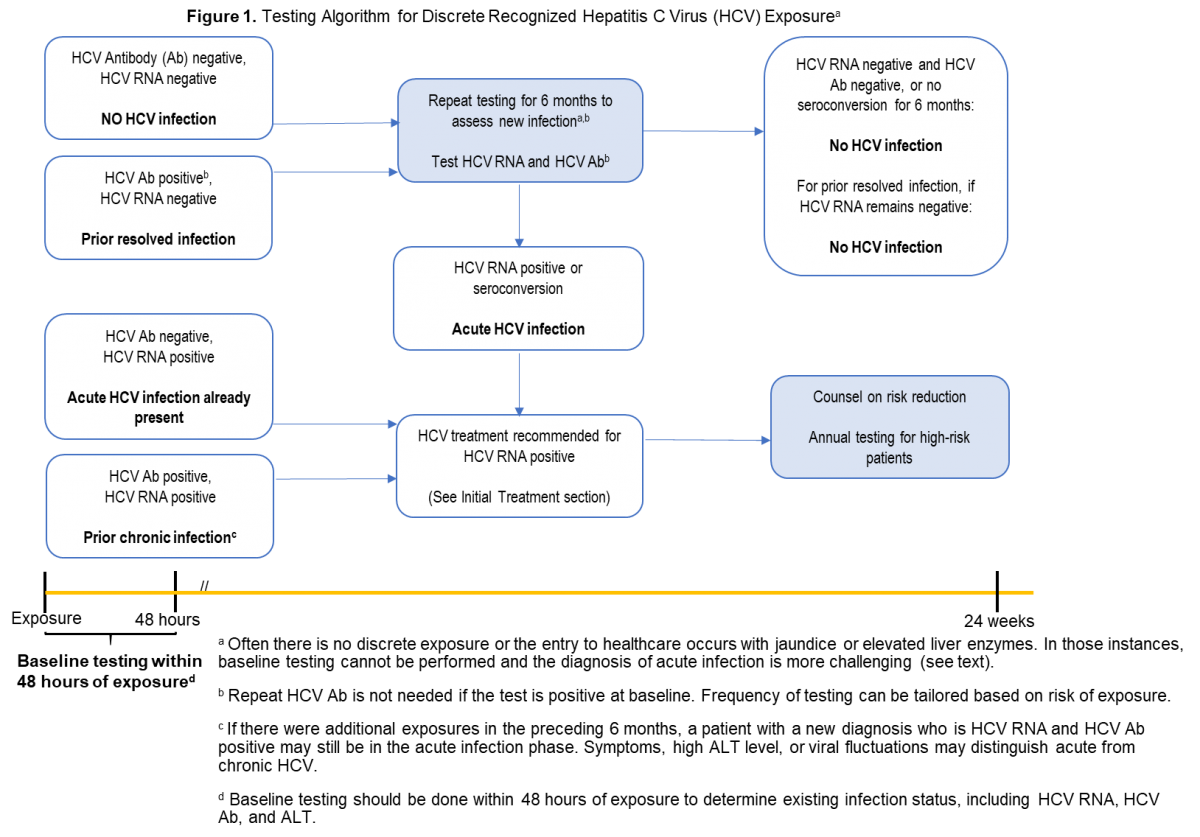
Management Of Acute Hcv Infection Hcv Guidance

Detection Of Hepatitis C Virus Rna In Blood And Saliva Of Transfusion Dependent Thalassemia Patients Diagnosed With Hepatitis C Hooshmand B Alavian Sm Kouhestani F Firouzmandi M Motamedian Sr Contemp Clin Dent

Evaluation Of The Cobas Hepatitis C Virus Hcv Taqman Analyte Specific Reagent Assay And Comparison To The Cobas Amplicor Hcv Monitor V2 0 And Versant Hcv na 3 0 Assays Journal Of Clinical Microbiology
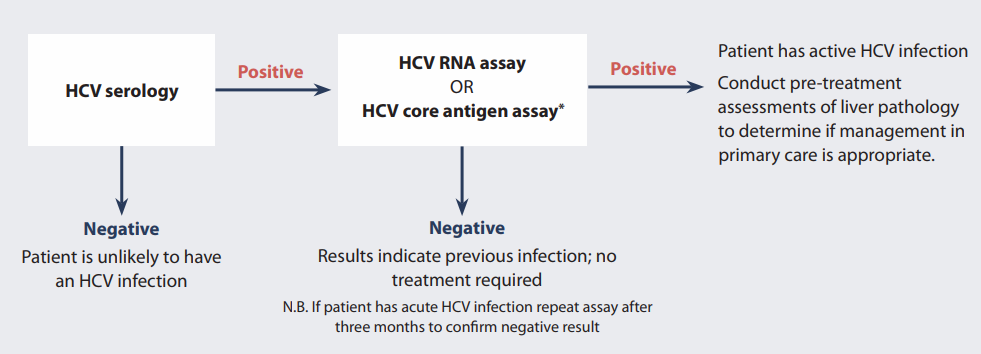
Testing For Hepatitis C Virus Hcv In Patients At High Risk Of Infection Bpacnz

Quantitative Nucleic Acid Amplification Methods And Their Implications In Clinical Virology

Hepatitis C
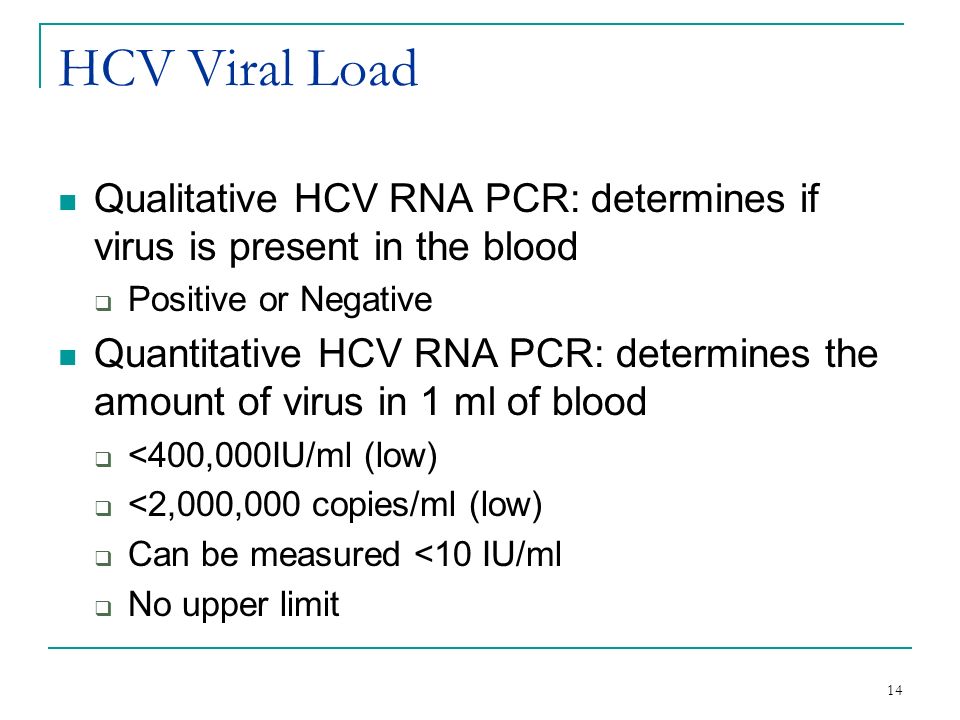
Interpreting Your Biopsy And Lab Results Ppt Video Online Download

The Evolution Of Occult Hepatitis C Virus After Immunosuppression In Advanced Ckd Patients Nefrologia English Edition

Hcv Core Antigen Comes Of Age A New Opportunity For The Diagnosis Of Hepatitis C Virus Infection In Clinical Chemistry And Laboratory Medicine Cclm Volume 56 Issue 6 18

Primary Screening Of Blood Donors By Nat Testing For Hcv Rna Development Of An In House Method And Results

Relationship Between Hcv Antigen Test And Quantitative Pcr For Hcv Rna Download Scientific Diagram
What Is The Quantitative Pcr For Hcv Rna What Is The Normal Range In Iu Ml Can It Be Curable If It Has A Higher Range Quora

Dominance Of Hepatitis C Virus Hcv Is Associated With Lower Quantitative Hepatitis B Surface Antigen And Higher Serum Interferon G Induced Protein 10 Levels In Hbv Hcv Coinfected Patients Clinical Microbiology And Infection

The Warde Report Issue 27 2 New Testing Options For Hepatitis C Virus
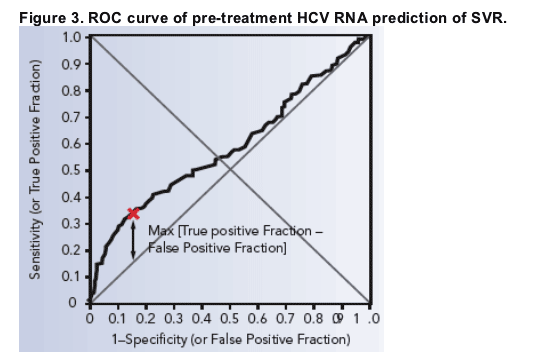
400 000 Iu Ml Is New Cut Off For Low Vs High Viral Load In Hcv
Ultrasensitive Hcv Rna Quantification In Antiviral Triple Therapy New Insight On Viral Clearance Dynamics And Treatment Outcome Predictors

Hepatitis C Viral Load Does Not Predict Disease Outcome Going Beyond Numbers

Performance Evaluation Of The Versant Hcv Rna Qualitative Assay By Using Transcription Mediated Amplification Journal Of Clinical Microbiology

Clin Mol Hepatol Clin Mol Hepatol Cmh Clinical And Molecular Hepatology 2287 2728 2287 285x The Korean Association For The Study Of The Liver 10 3350 Cmh 14 4 368 Original Article Predictors Of Spontaneous Viral Clearance And Outcomes Of
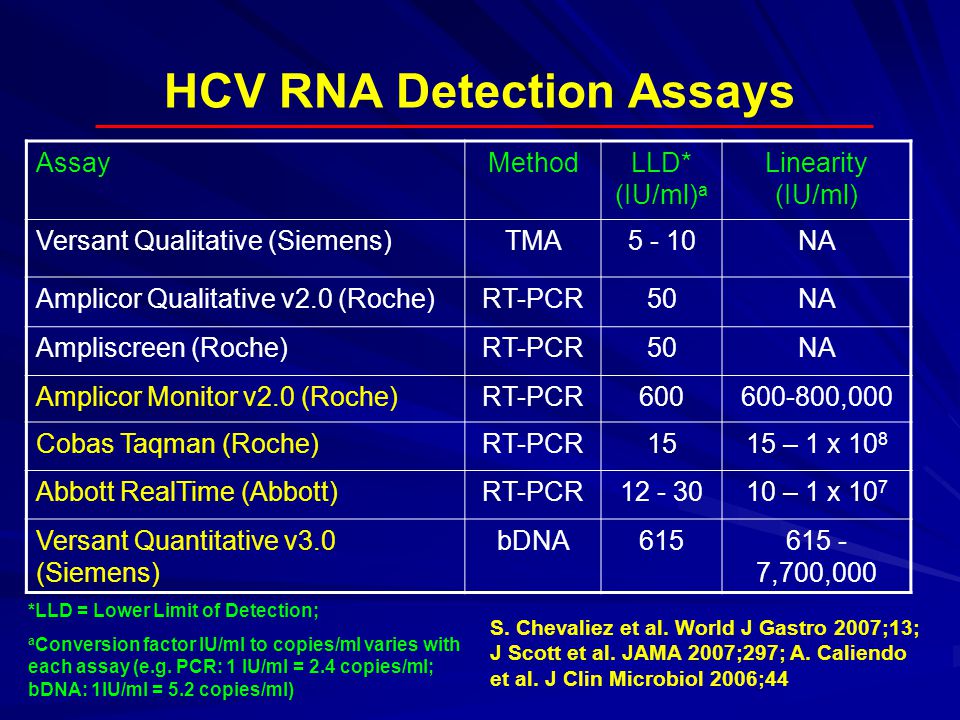
Laboratory Diagnostics In Hepatitis Ppt Video Online Download
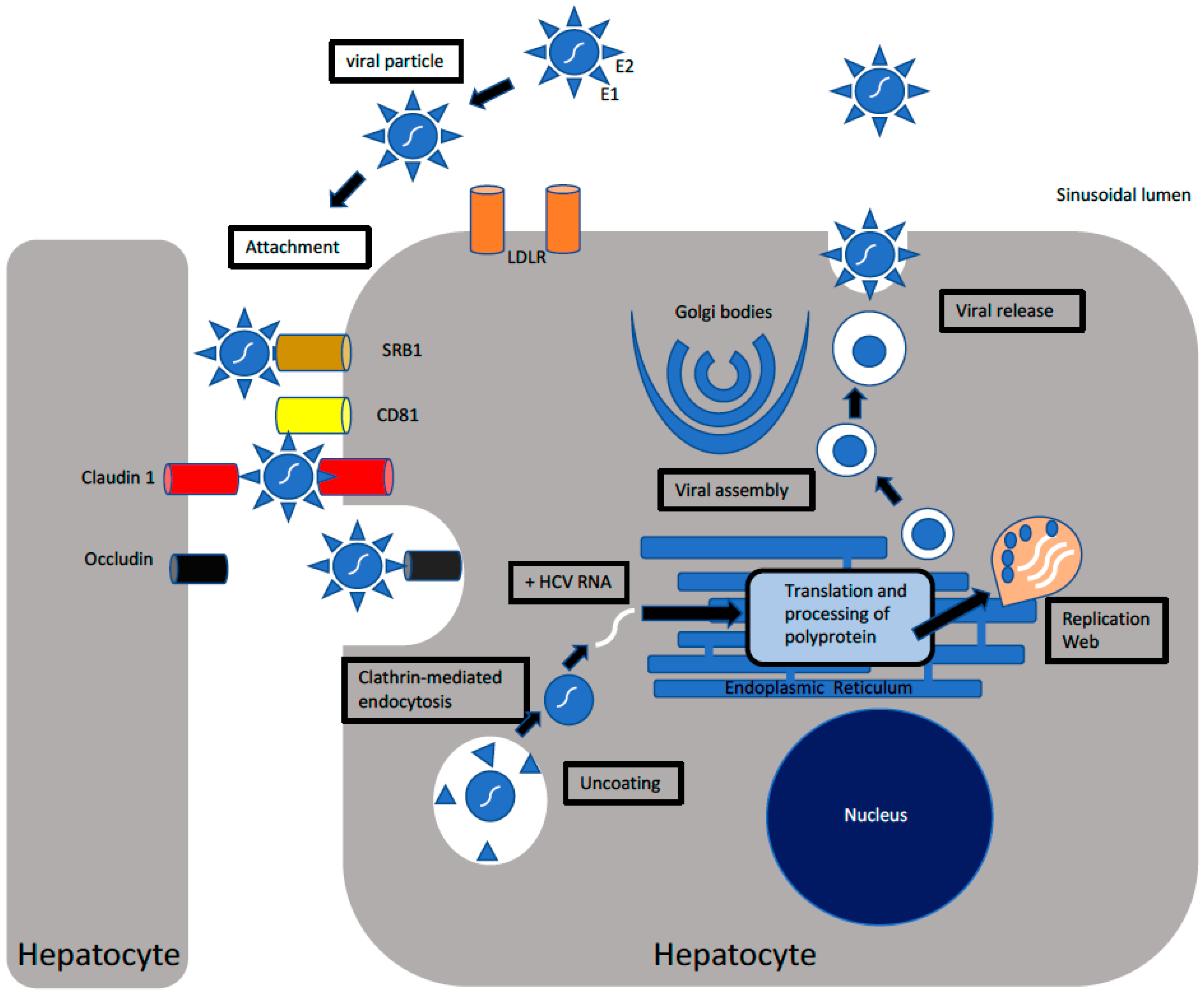
Cells Free Full Text Hepatitis C Virus Infection Host Virus Interaction And Mechanisms Of Viral Persistence Html
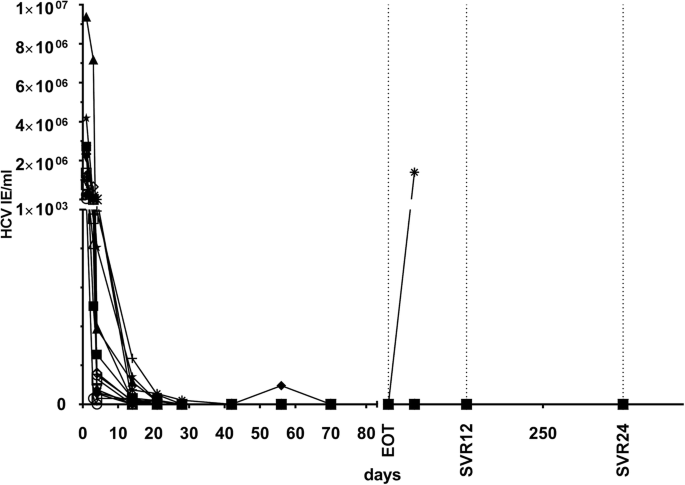
A Prospective Study Of Daclatasvir And Sofosbuvir In Chronic Hcv Infected Kidney Transplant Recipients Bmc Nephrology Full Text

Dynamics Of Hepatitis C Virus Infection Annals Of Hepatology
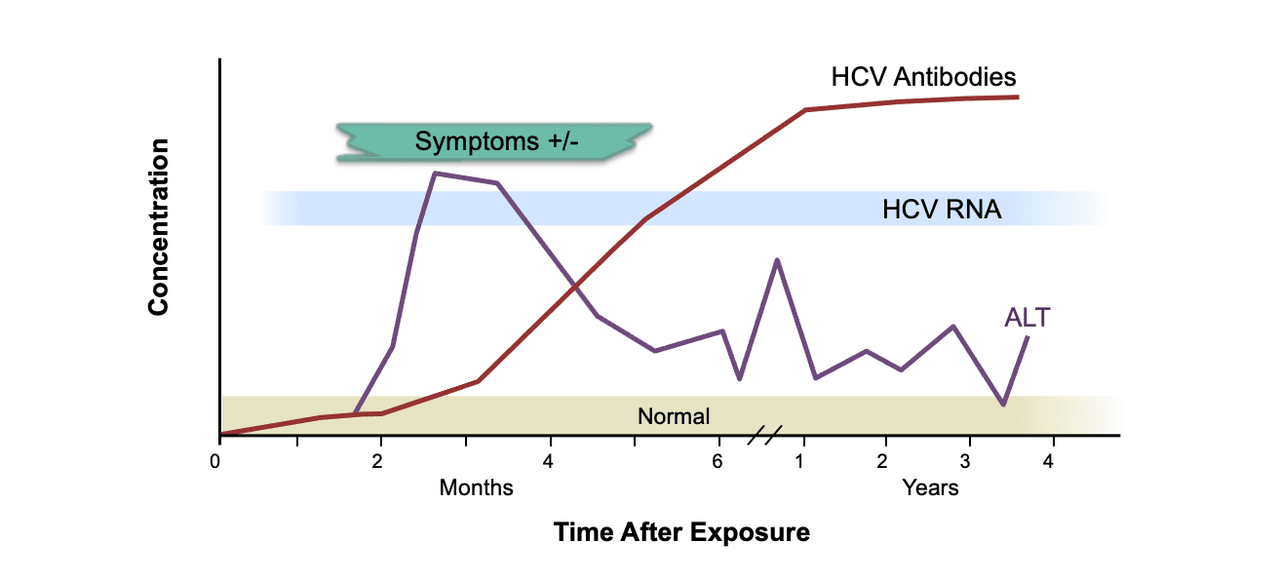
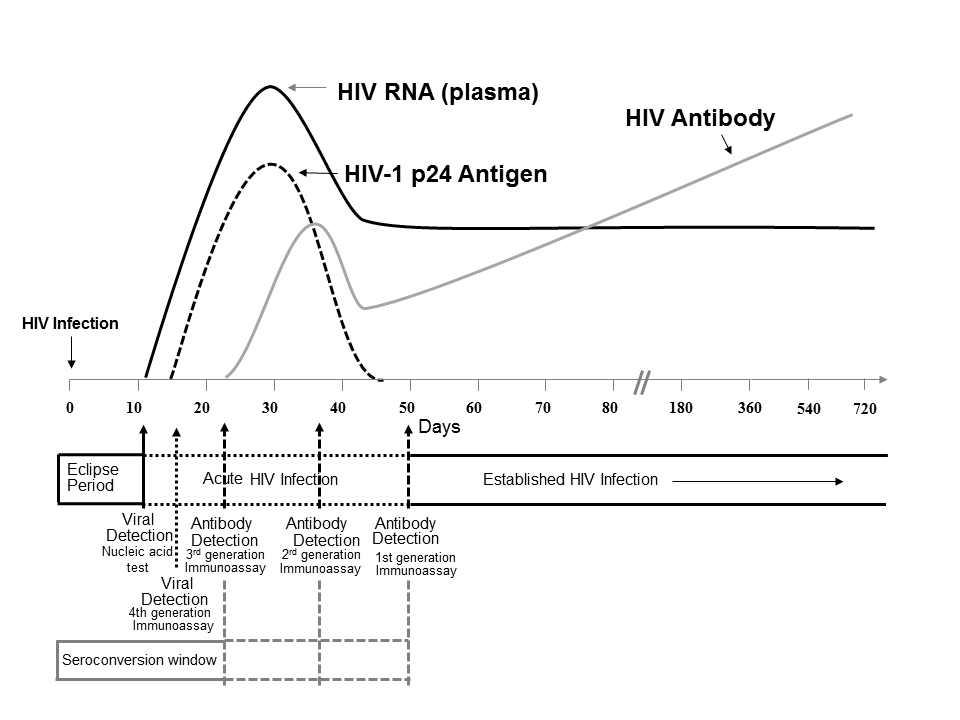
Core Concepts Diagnosis Of Acute Hcv Infection Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online
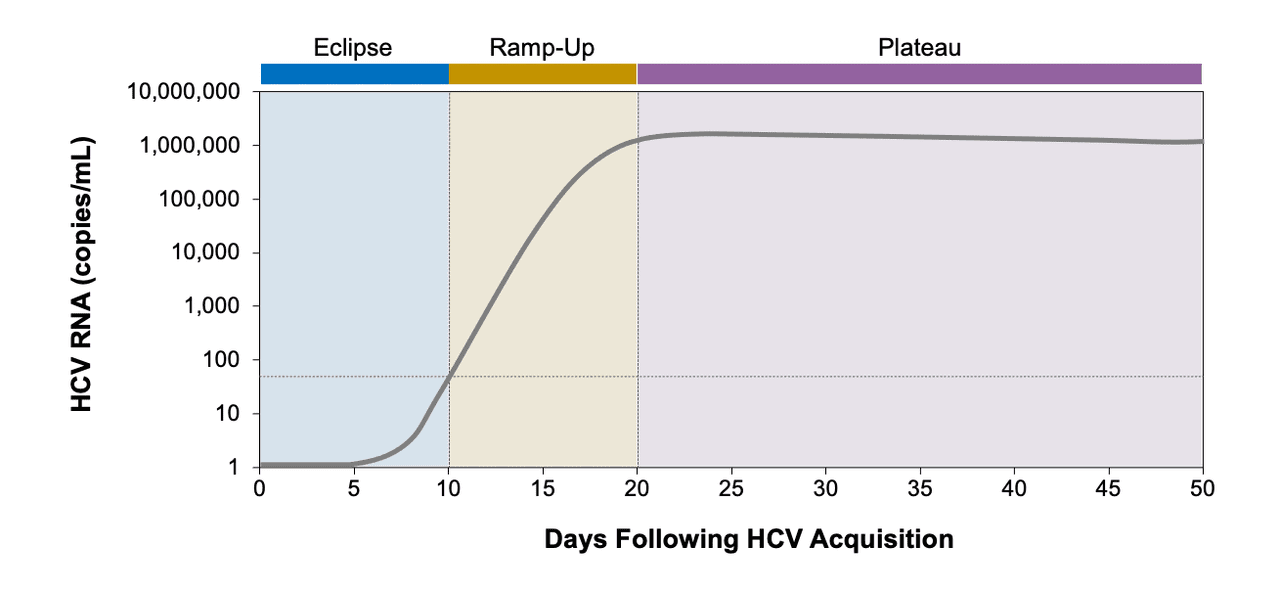
Core Concepts Diagnosis Of Acute Hcv Infection Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online

Utility Of Routine Real Time Quantitative Pcr Monitoring Of Hcv Infection In Haemodialysis Patients Datta S Goel N Wattal C Indian J Med Microbiol

Interferon L3 Gene Il28b Is Associated With Spontaneous Or Treatment Induced Viral Clearance In Hepatitis C Virus Infected Multitransfused Patients With Thalassemia Biswas 17 Transfusion Wiley Online Library

Estimation Of Hcv Viral Load And Liver Enzymes Among Different Patients Groups Of District Gujrat Pakistan
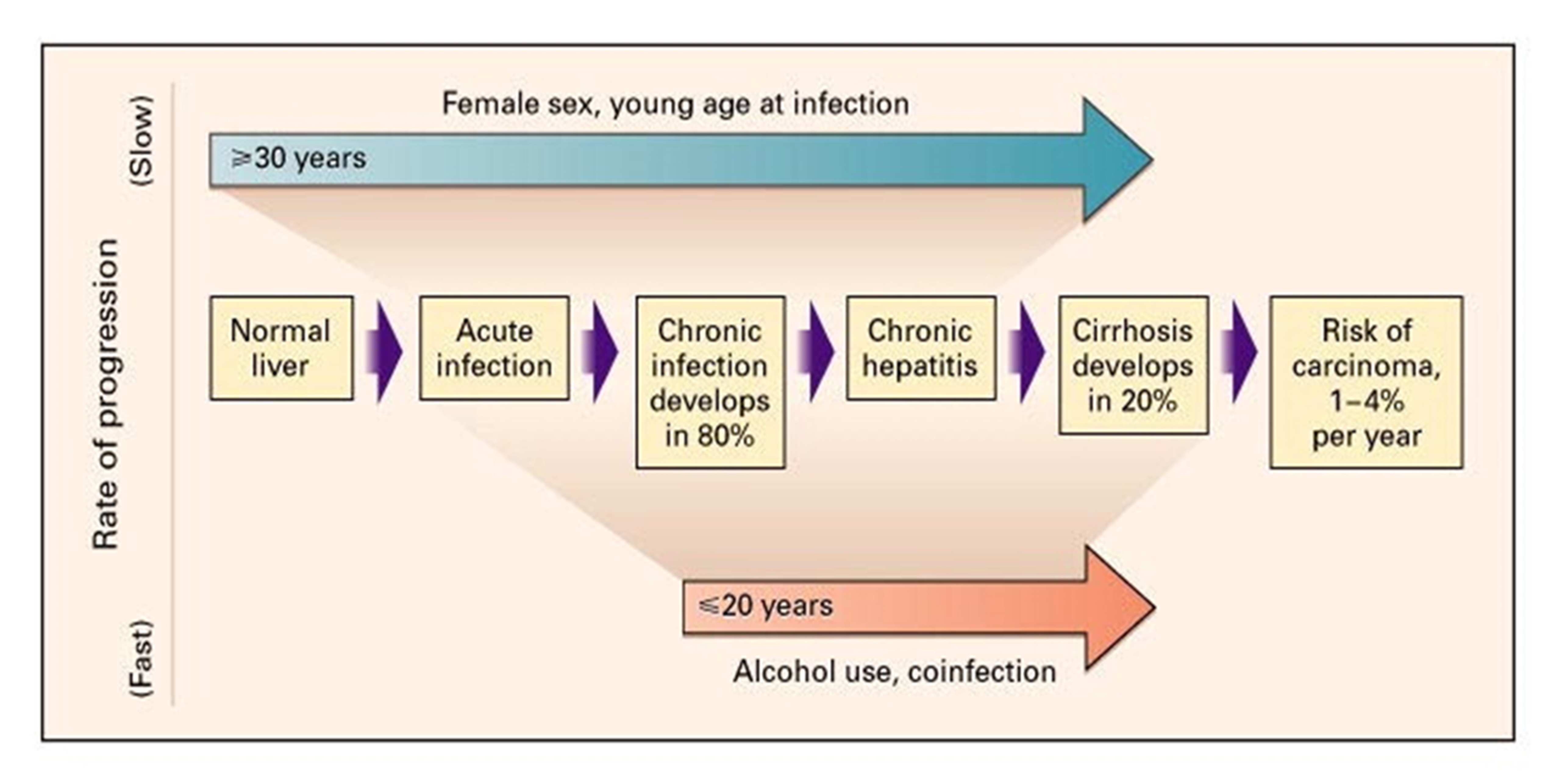
Hepatitis C
Q Tbn 3aand9gcr8ds1e Y Utwhec7dq4arutlwrcesksyitxirq Wc Pwiogibt Usqp Cau

Primary Screening Of Blood Donors By Nat Testing For Hcv Rna Development Of An In House Method And Results
Cepheid Hepatitis C Hcv Molecular Test Xpert Hcv Viral Load
Module 4 The Main Types Of Test Relevant To The Diagnosis And Management Of Hcv
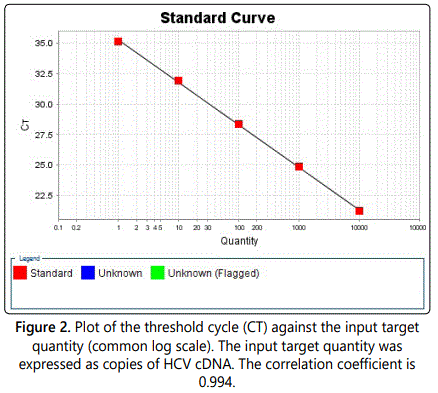
Determination Of Hcv Genotypes And Viral Loads In Chronic Hepatic Sudanese Infected Patients Madridge Publishers

Information On Hepatitis C Test Results Hep
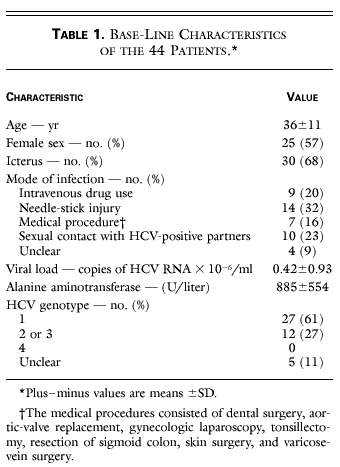
Treatment Of Acute Hepatitis C With Interferon Alfa 2b

A Case Report Of Sofosbuvir And Daclatasvirto Treat A Patient With Acute Hepatitis C Virus Genotype 2 Monoinfection Abstract Europe Pmc
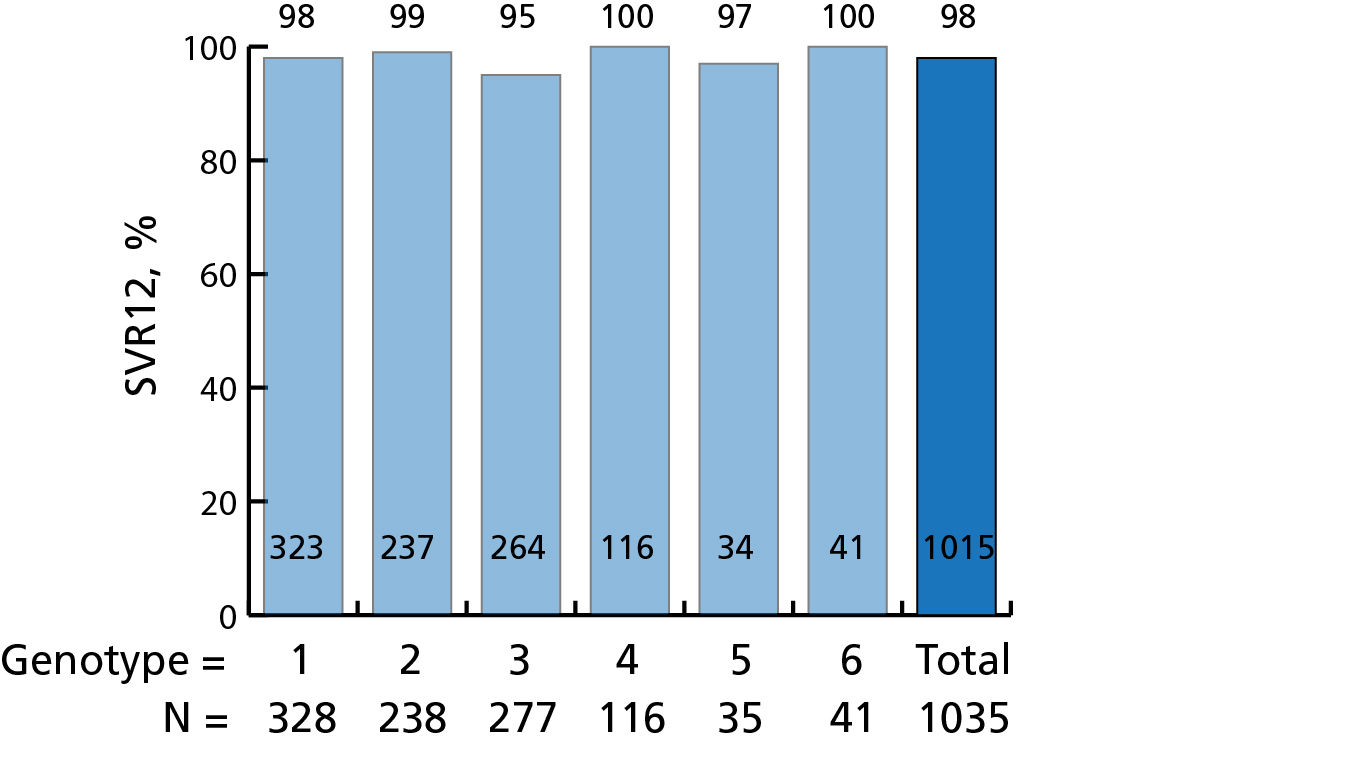
Hepatitis C

A Mathematical Model Of Hepatitis C Virus Dynamics In Patients With High Baseline Viral Loads Or Advanced Liver Disease Gastroenterology

Modeling Of Patient Virus Titers Suggests That Availability Of A Vaccine Could Reduce Hepatitis C Virus Transmission Among Injecting Drug Users Science Translational Medicine

Serum And Liver Hcv Rna Levels In Patients With Chronic Hepatitis C Correlation With Clinical And Histological Features Gut
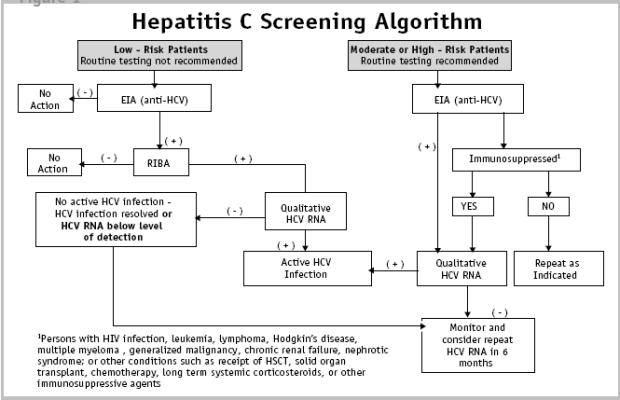
Clinical Guidelines For The Medical Management Of Hepatitis C

Hepatitis C Virus Total Antibody Hcv Ab Purpose Normal Range Of Results 1mg

Incidentally Detected Asymptomatic Hepatitis C Virus Infection With Significant Fibrosis Possible Impacts On Management Gupta Rk Sakhuja P Majumdar K Ali S Srivastava S Sachdeva S Sharma Puri As Indian

Hepatitis C A Review For Primary Care Physicians Cmaj

Predictors Of Sustained Virological Response To Therapy With Pegylated Interferon Plus Ribavirin In Hepatitis C Virus Patients

Adaptation Of Hepatitis C Virus To Interferon Lambda Polymorphism Across Multiple Viral Genotypes Elife

Evaluation Of The Cobas Hcv Test For Quantifying Hcv Rna In Dried Plasma Spots Collected Using The Cobas Plasma Separation Card Sciencedirect
Www Splcenter Org Sites Default Files D6 Legacy Files Baker V Campbell Hcv2 Pdf

Portal Hypertension Does Not Preclude The Efficacy Of Direct Acting Anti Hepatitis C Viral Therapy Clinmed International Library Journal Of Clinical Gastroenterology And Treatment
Patterns Of Hepatitis C Virus Rna Levels During Acute Infection The Inc3 Study

What Is Viral Load Livers With Life

Modeling Of Patient Virus Titers Suggests That Availability Of A Vaccine Could Reduce Hepatitis C Virus Transmission Among Injecting Drug Users Science Translational Medicine

Hcv Rna Pcr What To Know About Hepatitis C Testing



