Hcv Rna Quantitative Test Price
Cepheid Hepatitis C Hcv Molecular Test Xpert Hcv Viral Load

Performance Comparison Of New Generation Hcv Core Antigen Test Versus Hcv Rna Test In Management Of Hepatitis C Virus Infection Transfusion And Apheresis Science

Evaluation Of The Cobas Hepatitis C Virus Hcv Taqman Analyte Specific Reagent Assay And Comparison To The Cobas Amplicor Hcv Monitor V2 0 And Versant Hcv na 3 0 Assays Journal Of Clinical Microbiology
Gale Onefile Health And Medicine Document Financial Impact Of Two Different Ways Of Evaluating Early Virological Response To Peginterferon Alpha 2b Plus Ribavirin Therapy In Treatment Naive Patients With Chronic Hepatitis C Virus

Qualitative Detection Of Hepatitis C Virus Rna Comparison Of Analytical Sensitivity Clinical Performance And Workflow Of The Cobas Amplicor Hcv Test Version 2 0 And The Hcv Rna Transcription Mediated Amplification Qualitative Assay

Diagnosis And Management Of Hepatitis C American Family Physician
HCV RNA BY REAL TIME PCR QUANTITATIVE Test Cost.

Hcv rna quantitative test price. The quantitative HCV RNA tests measure the amount of hepatitis C virus in the blood. This test quantifies HCV RNA of free HCV virions in serum / plasma. Hepatitis C Test Cost will be between $24.00 and $109.00.
HEPATITIS C VIRAL RNA (HCV RNA) QUANTITATIVE ULTRA:. Hepatitis C is a bloodborne disease and exposure to the blood of an. The following table shows the HCV RNA test cost at 3 of our partner laboratories (CLIA – Certified) network located across the U.S.
Hepatitis C Test Cost max is in Private MD (Hepatitis C Antibody with Reflex) with price $192.99. HCV RNA BY REAL TIME PCR QUANTITATIVE genetic dna test done through Real Time PCR sample type Peripheral blood/Plasma/Serum/Tissue/Body Fluids/Amniotic fluid/Cervical swab EDTA vacutainer – 3ml peripheral blood,plasma,serum Sterile container with Normal saline – tissues Sterile container – Body fluids and Amniotic fluid Sterile Swab – cervical material Ambient reporting time for results 3-4 days 5800. Reportable range is 15 to 100,000,000 IU/mL (1.18-8.00 log IU/mL).
The result will be an exact number, such as "1,215,422 IU/L." Many people refer to the quantitative measurement as the hepatitis C "viral load." Viral load tests are used to confirm active hepatitis C infection and are used during treatment to help determine. Other things to know:. Hepatitis C Test Cost minimal is in HealthLabs (Hepatitis C Virus (HCV) Antibody Test) with price $24.00.
Hepatitis C test cost with insurance. If a qualitative RNA test is positive (detected), then it is confirmed that the patient has chronic hepatitis C. Hepatitis C is a liver disease that is caused by the hepatitis C virus (HCV).
Separate serum / plasma aseptically within 6 hours. 3 mL (2 mL min.) serum from 2 SST's OR 3 mL (2 mL min.) plasma from 2 Lavender Top (EDTA) tubes. The "qualitative" test is more accurate than the "quantitative" test because qualitative tests are able to detect very low levels of the virus.
Diseases of Liver :. $295.99 Quest Diagnostics Price:. Sample Tue / Fri by 11 am;.
Explanation of test results:. Hepatitis C Viral RNA, Quantitative, Real-Time PCR - Useful in monitoring therapy and/or disease progression. $ 344.49 Click to view our Quest Diagnostics tests or click Add to Cart below to buy this test from LabCorp.
Report Thu / Mon:. HCV RNA is a marker of viral replication and persistence is. Kleiber J, Walter T, Haberhausen G, Tsang S, Babiel R, Rosenstraus M.
This test is intended for use as an aid in management of HCV infected patients and is not intended for use in the initial diagnosis or confirmation of HCV infection.
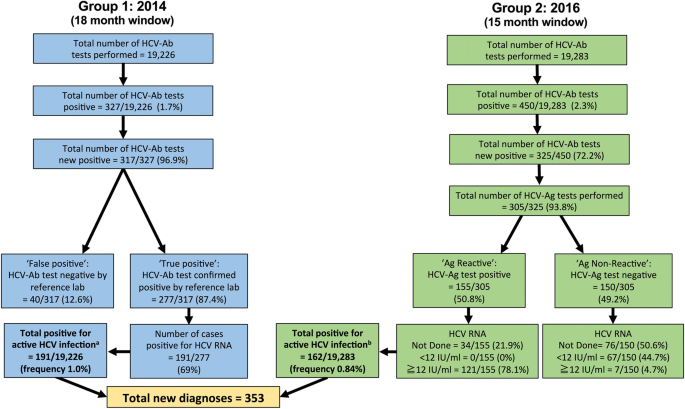
Hepatitis Virus Hcv Diagnosis And Access To Treatment In A Uk Cohort Bmc Infectious Diseases Full Text
Www Journalofinfection Com Article S0163 4453 19 6 Pdf

Evaluation Of Quantitative Real Time Pcr As A Hepatitis C Virus Supplementary Test After Riba Discontinuation Abstract Europe Pmc

Pdf Introduction Of An Automated System For The Diagnosis And Quantification Of Hepatitis B And Hepatitis C Viruses Semantic Scholar
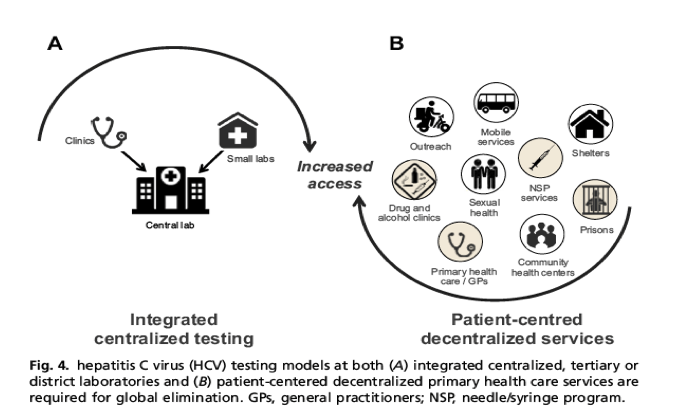
Hcv Test Treat Hepatitis C Virus Diagnosis And The Holy Grail
Q Tbn 3aand9gcsmixdydp9zxbvoxpm6gpkob4asxbgucvxco9h 6ujissfkp0ve Usqp Cau
Www Who Int Hepatitis Publications Annex 4 7 Pdf Ua 1

Jrp Decentralized Community Based Hepatitis C Point Of Care Testing And Direct Acting Antiviral Treatment For People Who Inject Drugs And The General Population In Myanmar Protocol For A Feasibility Study Draper Jmir

Systematic Reviews And Evidence Summaries Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf
View Of Is Hcv Core Antigen A Reliable Marker Of Viral Load An Evaluation Of Hcv Core Antigen Automated Immunoassay Annals Of Gastroenterology
Files Labcorp Com Labcorp D8 02 L 0417 2 Pdf

Hcv Rna Pcr What To Know About Hepatitis C Testing

Primary Screening Of Blood Donors By Nat Testing For Hcv Rna Development Of An In House Method And Results
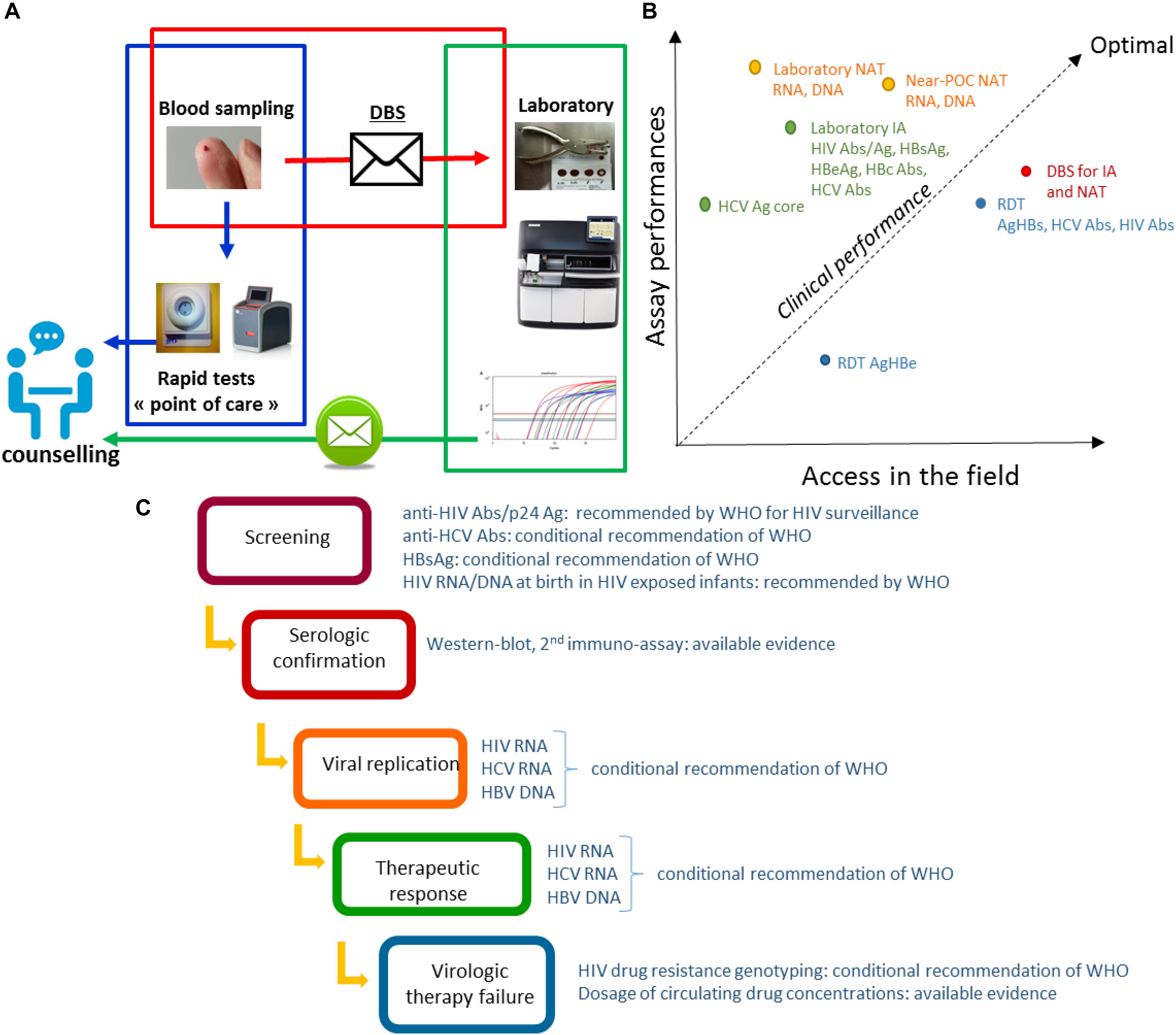
Frontiers Dried Blood Spot Tests For The Diagnosis And Therapeutic Monitoring Of Hiv And Viral Hepatitis B And C Microbiology
2
Ultrasensitive Hcv Rna Quantification In Antiviral Triple Therapy New Insight On Viral Clearance Dynamics And Treatment Outcome Predictors

A Rational Use Of Laboratory Tests In The Diagnosis And Management Of Hepatitis C Virus Infection Sciencedirect

Full Text Expert Opinion On The Management Of Hepatitis C Infection In Kuwait Hmer
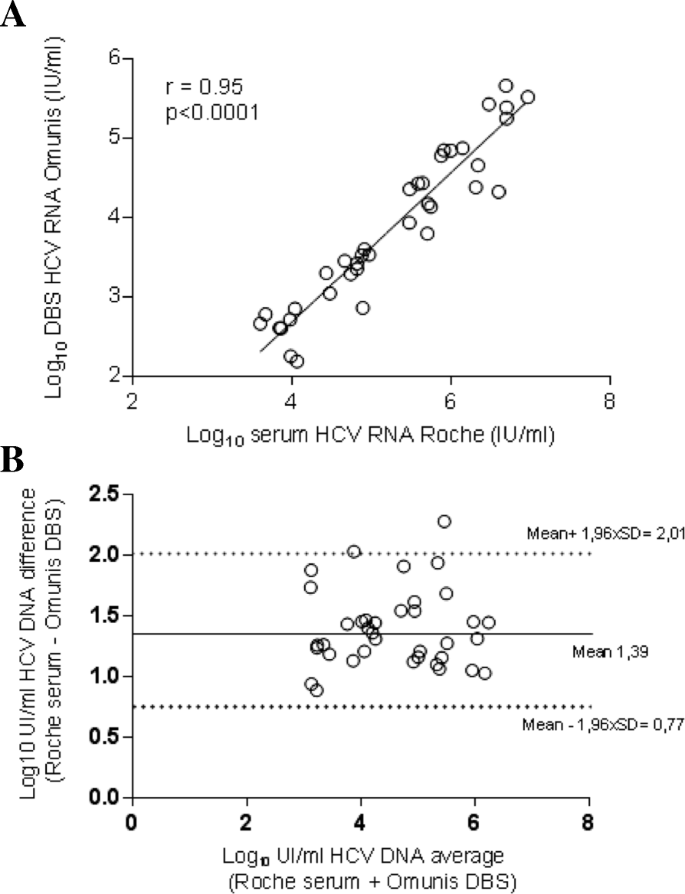
Confirmation Of Hcv Viremia Using Hcv Rna And Core Antigen Testing On Dried Blood Spot In Hiv Infected Peoples Who Inject Drugs In Vietnam Bmc Infectious Diseases Full Text

Diagnosis And Management Of Hepatitis C American Family Physician

Hepatitis C Testing Hepatitis C Treatment
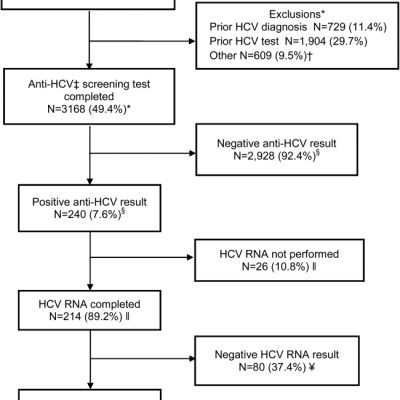
Baby Boomer Hcv Screening And Care Journal Of Hospital Medicine

The Warde Report Issue 27 2 New Testing Options For Hepatitis C Virus

Ampiprobe Hcv Enzo Clinical Labs

Evaluation Of Assay Methods And False Positive Results In The Laboratory Diagnosis Of Hepatitis C Virus Infection Insight Medical Publishing

Bioinformatics Analysis Of Quantitative Pcr And Reverse Transcription Pcr In Detecting Hcv Rna Bentham Science

Genotyping Diagnostic Methods For Hepatitis C Virus A Need Of Low Resource Countries Kumar A Rajput Mk Paliwal D Yadav A Chhabra R Singh S Indian J Med Res

Full Text The Very Rapid And The Ultra Rapid Virologic Response To Two Treatment Dddt

Primary Screening Of Blood Donors By Nat Testing For Hcv Rna Development Of An In House Method And Results

Cepheid Hepatitis C Hcv Molecular Test Xpert Hcv Viral Load

Real Time Pcr Assays For Hepatitis C Virus Hcv Rna Quantitation Are Adequate For Clinical Management Of Patients With Chronic Hcv Infection Journal Of Clinical Microbiology
2

The Warde Report Issue 27 2 New Testing Options For Hepatitis C Virus

Diagnostic Performance Of Qualitative Vs Quantitative Hcv Rna Nat In Download Table
Cobas Ampliprep Cobas Taqman Hcv Test V2 0 Qualitative And Quantitative

Cost Effectiveness Of Universal Screening For Hepatitis C Virus Infection In The Era Of Direct Acting Pangenotypic Treatment Regimens Clinical Gastroenterology And Hepatology
Www Who Int Hepatitis Publications Annex 4 9 Pdf Ua 1

Cost Effectiveness Of Strategies For Testing Current Hepatitis C Virus Infection Chapko 15 Hepatology Wiley Online Library
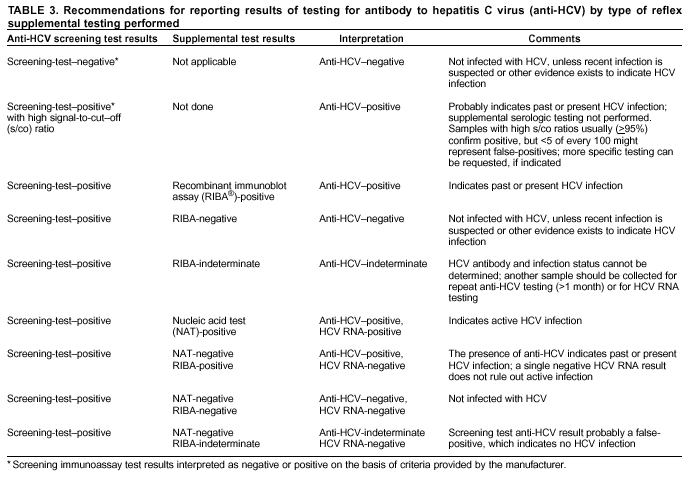
Guidelines For Laboratory Testing And Result Reporting Of Antibody To Hepatitis C Virus
View Of Is Hcv Core Antigen A Reliable Marker Of Viral Load An Evaluation Of Hcv Core Antigen Automated Immunoassay Annals Of Gastroenterology

Commercially Available Quantitative Real Time Pcr Based Hepatitis C Download Table
Www Who Int Hepatitis Publications Annex 4 7 Pdf Ua 1

Hepatitis C Virus Hcv Diagnosis Epidemiology And Access To Treatment In A Uk Cohort Biorxiv

Hepatitis C Virus Blood Test Quantitative Rna Pcr Antibody Test

Systematic Reviews And Evidence Summaries Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf

Field Evaluation Of Genexpert Cepheid Hcv Performance For Rna Quantification In A Genotype 1 And 6 Predominant Patient Population In Cambodia Iwamoto 19 Journal Of Viral Hepatitis Wiley Online Library

Hepatitis C Virus Core Antigen A Simplified Treatment Monitoring Tool Including For Post Treatment Relapse Sciencedirect
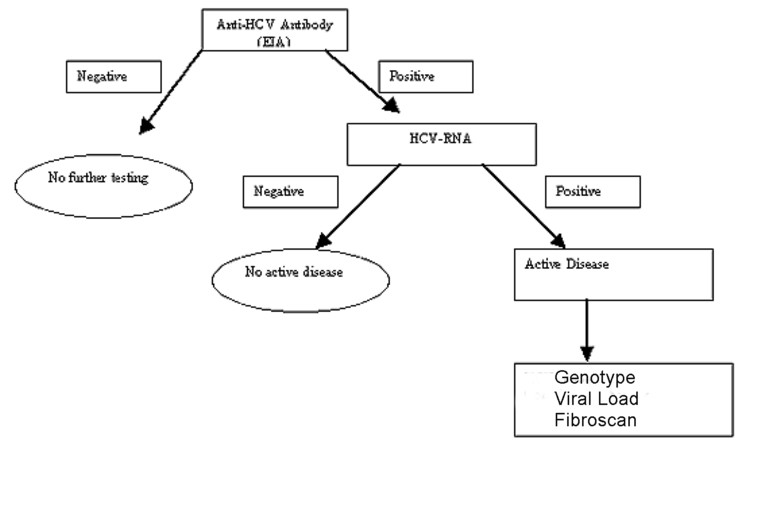
Hep C Tests Results Information Explanation And Costs

Cost Effectiveness Of Strategies For Testing Current Hepatitis C Virus Infection Chapko 15 Hepatology Wiley Online Library

Screening And Treatment Program To Eliminate Hepatitis C In Egypt Nejm
Q Tbn 3aand9gcrsms Ay5mzevkwxaobeqkxsgu16affteei6yznq0sr4xuu4ect Usqp Cau
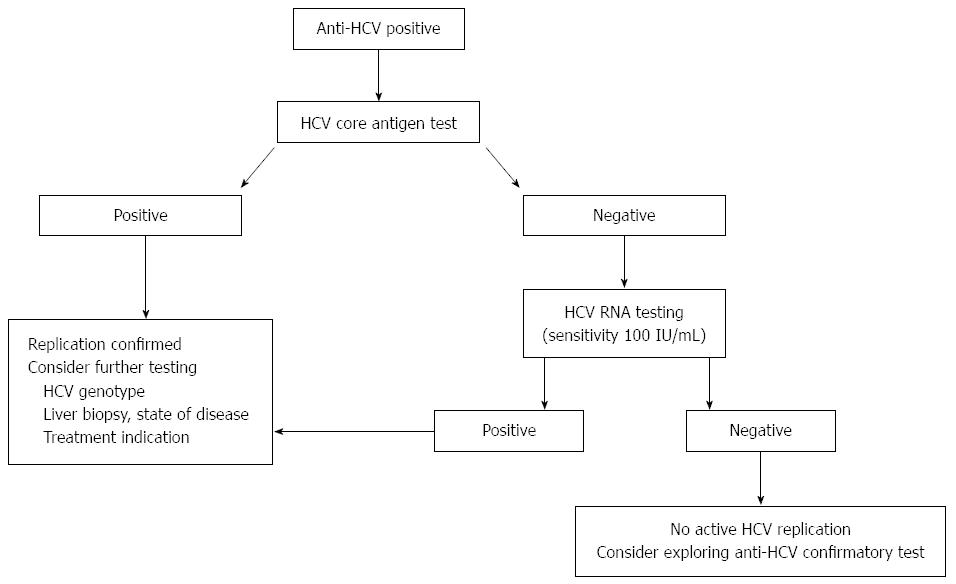
Hepatitis C Virus Core Antigen Testing Role In Diagnosis Disease Monitoring And Treatment

Core Concepts Hepatitis C Diagnostic Testing Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online
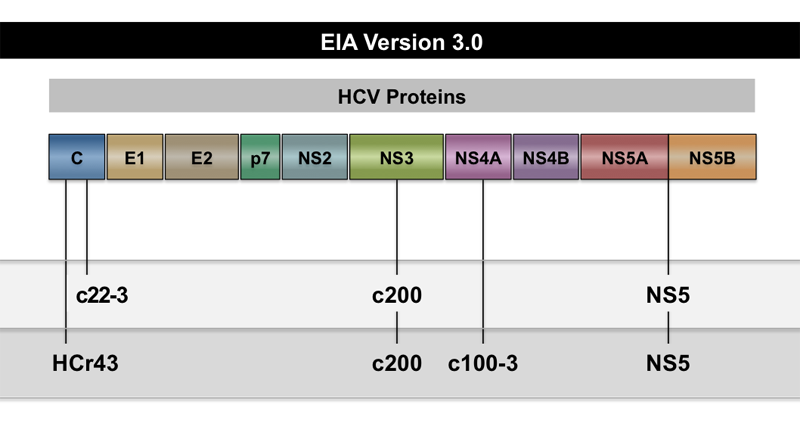
Core Concepts Hepatitis C Diagnostic Testing Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online

Hcv Core Antigen Comes Of Age A New Opportunity For The Diagnosis Of Hepatitis C Virus Infection In Clinical Chemistry And Laboratory Medicine Cclm Volume 56 Issue 6 18

Hepatitis C Virus Hcv Rna Viral Load Quantitative Online Booking With Price At Micron Laboratory Green Park New Delhi 3hcare
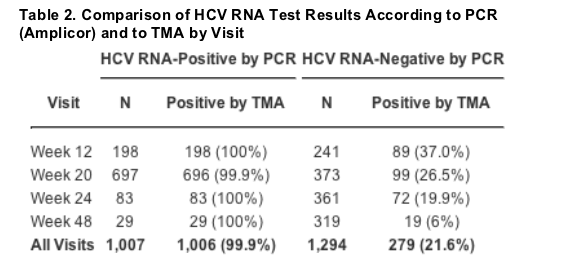
Hcv Rna Detection By Tma During The Hepatitis C Antiviral Long Term Treatment Against Cirrhosis Halt C Trial
Hcv Rna Pcr How This Hepatitis C Virus Test Works Results More
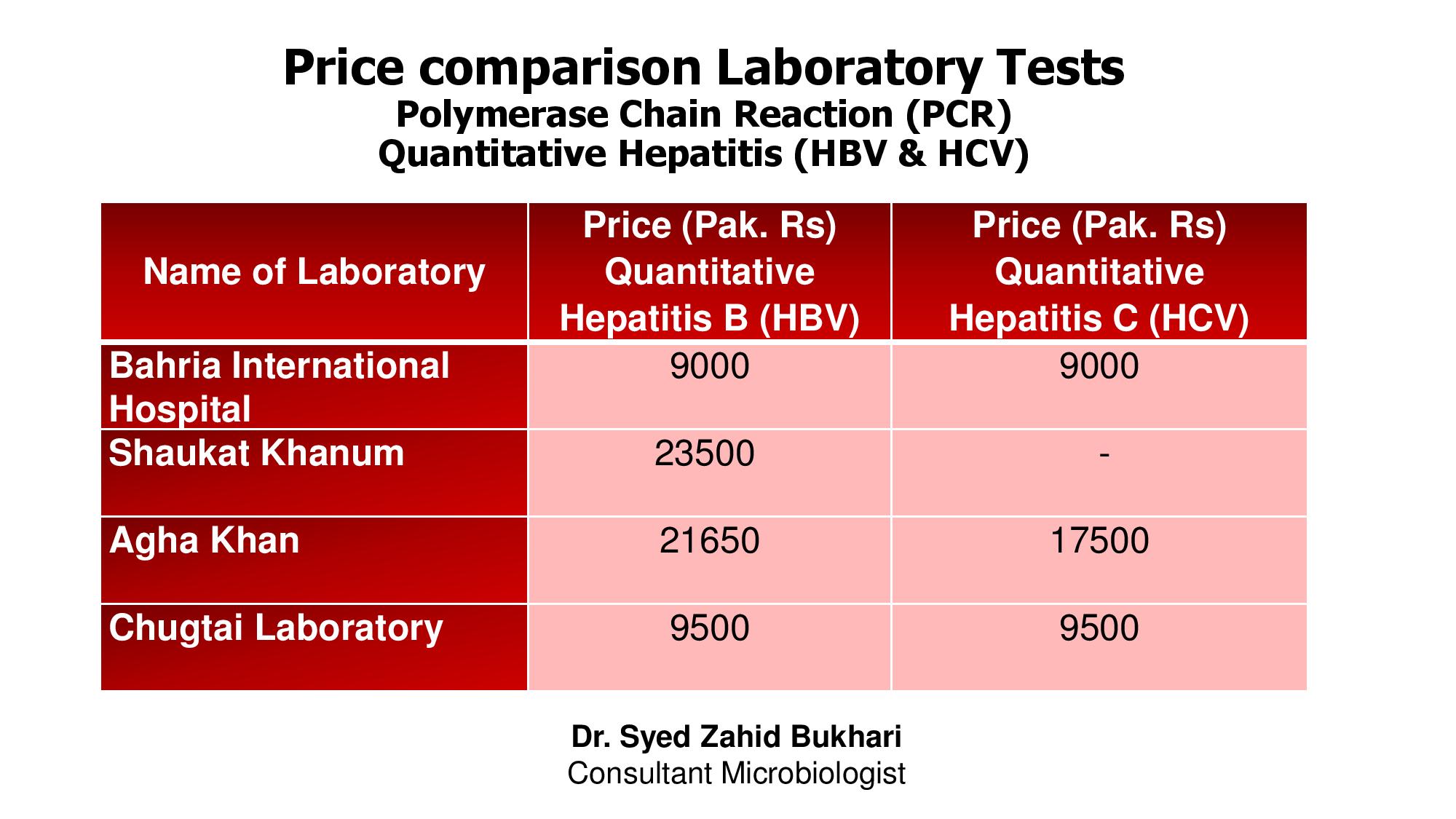
Price List Comparison Laboratory Tests Pcr Hbv Hcv Quantitative Bahria International Hospitals

Realtime Hcv Viral Load Assay Abbott Molecular
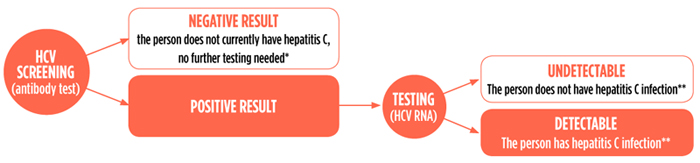
Hepatitis C Virus Hcv Diagnostics Fact Sheet Treatment Action Group

Hepatitis C Decode
Q Tbn 3aand9gcqacccheqifmas3nd08iuujpdddvwq9sw1u70zgark Lcvbxpxi Usqp Cau

Complete Std 10 Panel Test By Labtestingapi Issuu

Hcv Rna Quantitative Test Was Done In All Patients Prior To Treatment Download Table
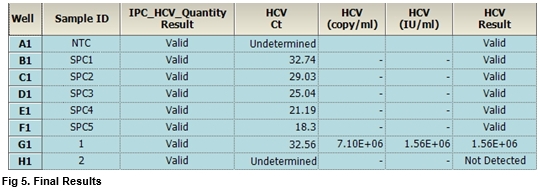
Accupower Hcv Quantitative Rt Pcr Kit

Hepatitis C Rna Test Hcv Rna Blood Test Accesa Labs
Hepatitis C Rna Assay Differences In Results Potential Implications For Shortened Therapy And Determination Of Sustained Virologic Response Scientific Reports

Prevalence Of Hepatitis C Virus Infection And Its Correlates In A Rural Area Of Southwestern China A Community Based Cross Sectional Study Bmj Open
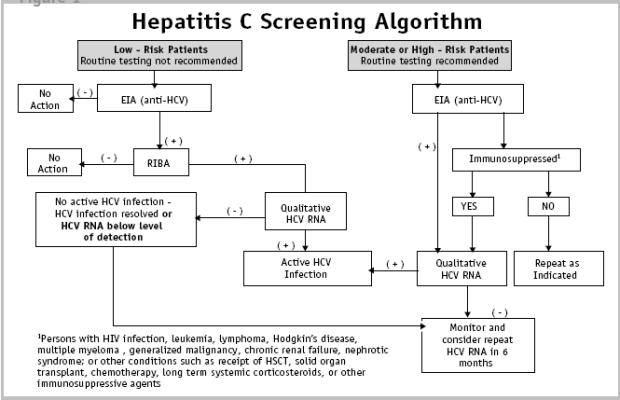
Clinical Guidelines For The Medical Management Of Hepatitis C
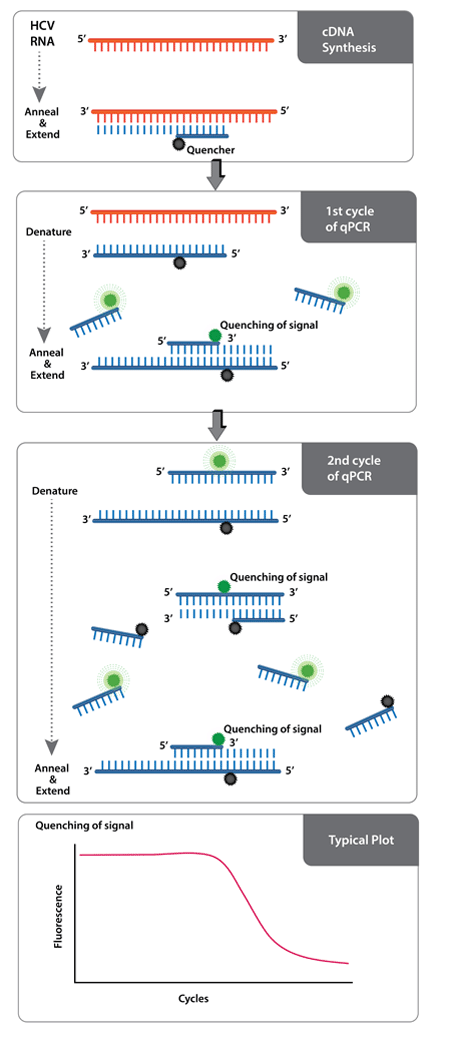
Ampiprobe Hcv Assay Kit Enz Gen0 Enzo Life Sciences

The Korean Journal Of Internal Medicine
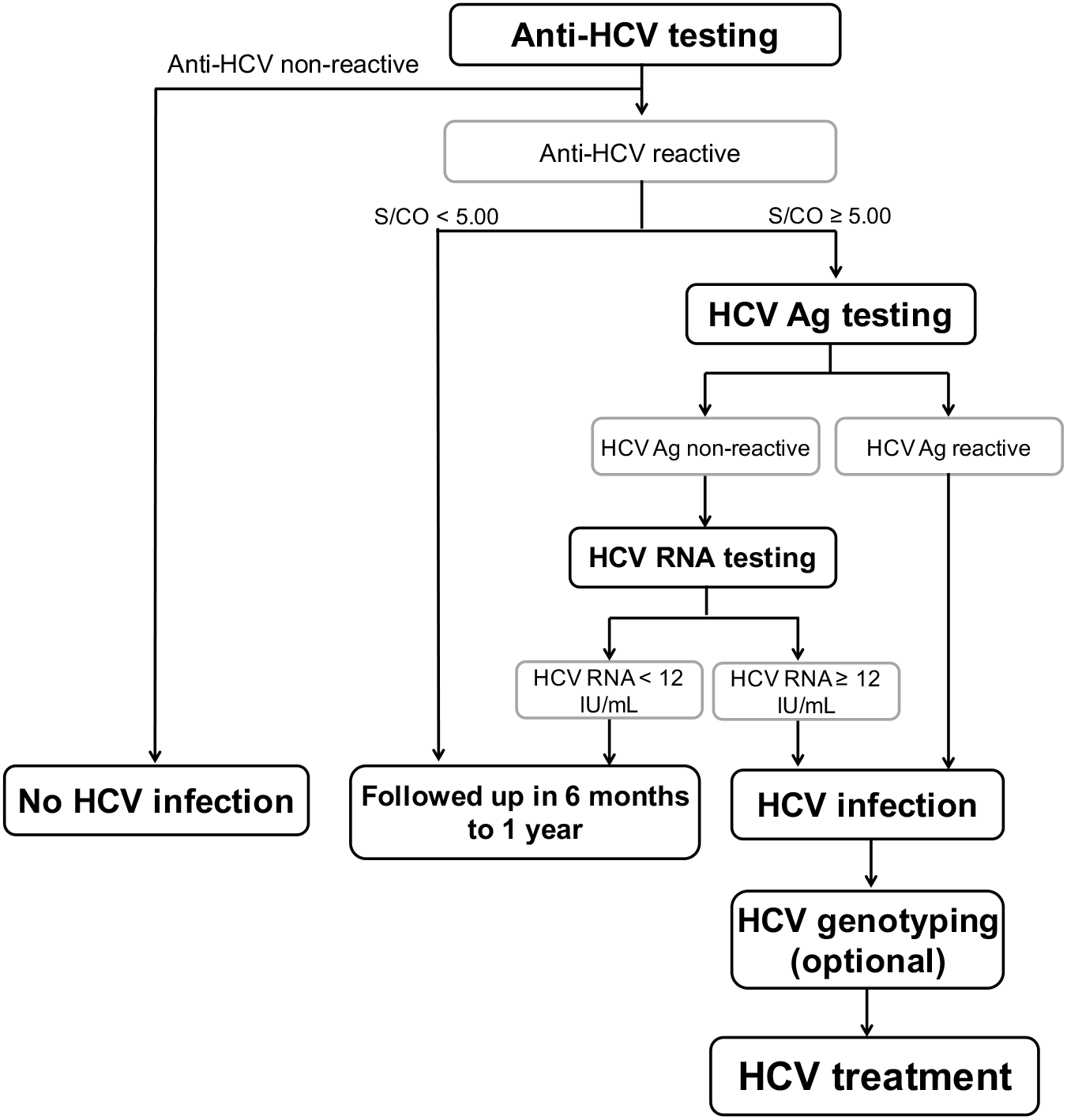
Hcv Core Antigen Is An Alternative Marker To Hcv Rna For Evaluating Active Hcv Infection Implications For Improved Diagnostic Option In An Era Of Affordable Daas Peerj
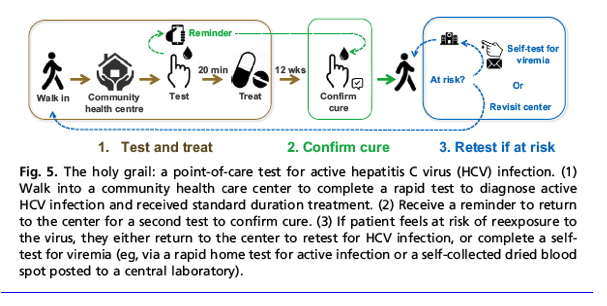
Hcv Test Treat Hepatitis C Virus Diagnosis And The Holy Grail
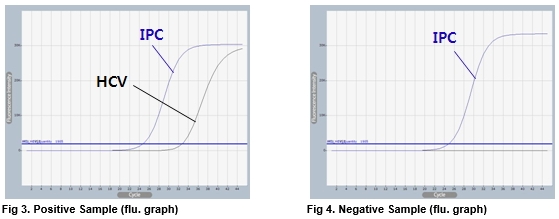
Accupower Hcv Quantitative Rt Pcr Kit

Hepatitis C Testing Lab Tests Online

Comparison Of Results From 142 Samples Obtained By The Versant Hcv Rna Download Scientific Diagram
Hepatitis C Test Cost Quest Find Lab Tests Online
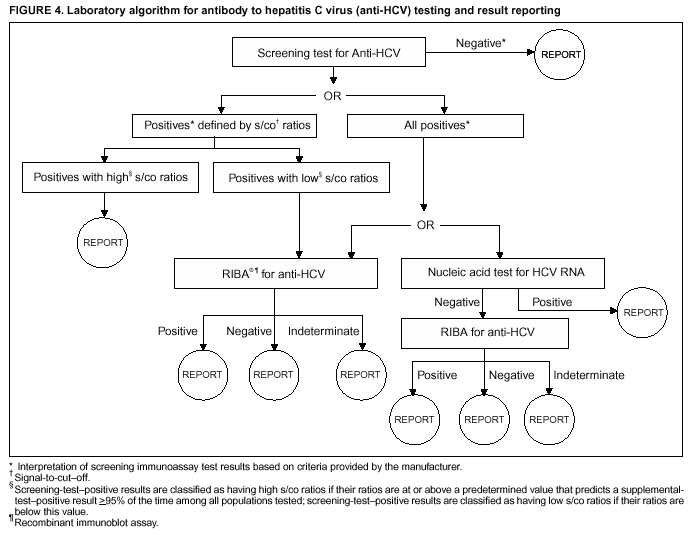
Guidelines For Laboratory Testing And Result Reporting Of Antibody To Hepatitis C Virus

Hepatitis C Virus Hcv Diagnostics Fact Sheet Treatment Action Group
The Role Of Hepatitis C Virus Core Antigen Testing In The Era Of Direct Acting Antiviral Therapies What We Can Learn From The Protease Inhibitors

Hepatitis C
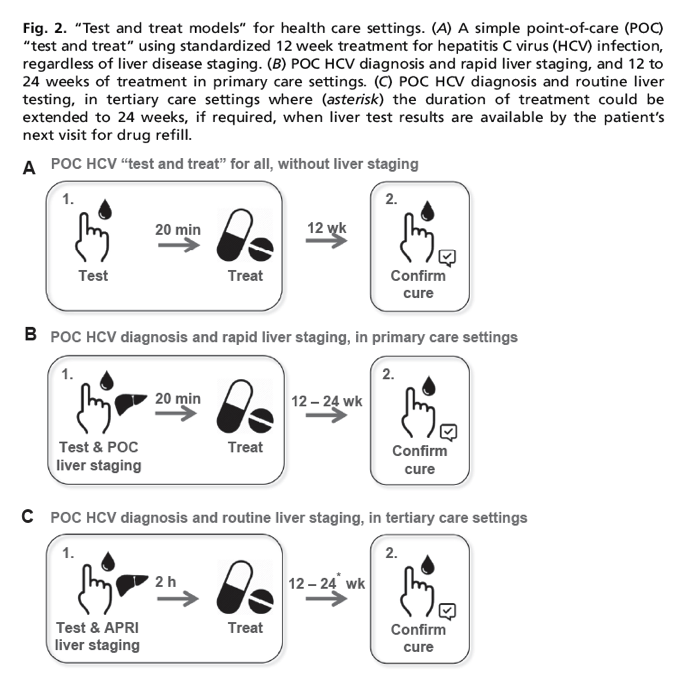
Hcv Test Treat Hepatitis C Virus Diagnosis And The Holy Grail

Hepatitis C Virus Hcv Diagnosis Epidemiology And Access To Treatment In A Uk Cohort Biorxiv

Quest Diagnostics Hep C Screening And Diagnosis

Get Lowest Hepatitis C Hcv Test Cost 24 Book Online Now

Hcv Rna Quantitative Test Was Done In All Patients Prior To Treatment Download Table
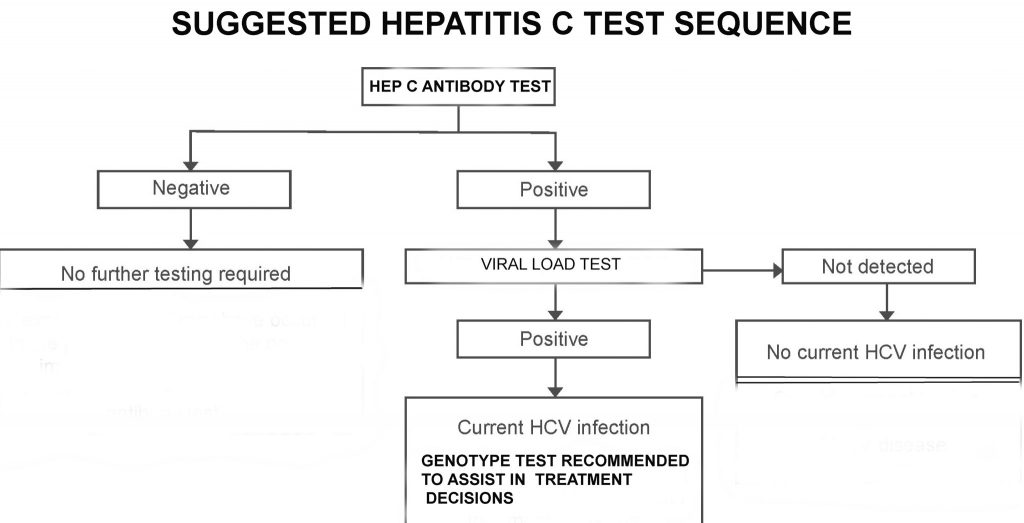
Hepatitis C Testing Hepatitis C Treatment
Systematic Reviews And Evidence Summaries Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf
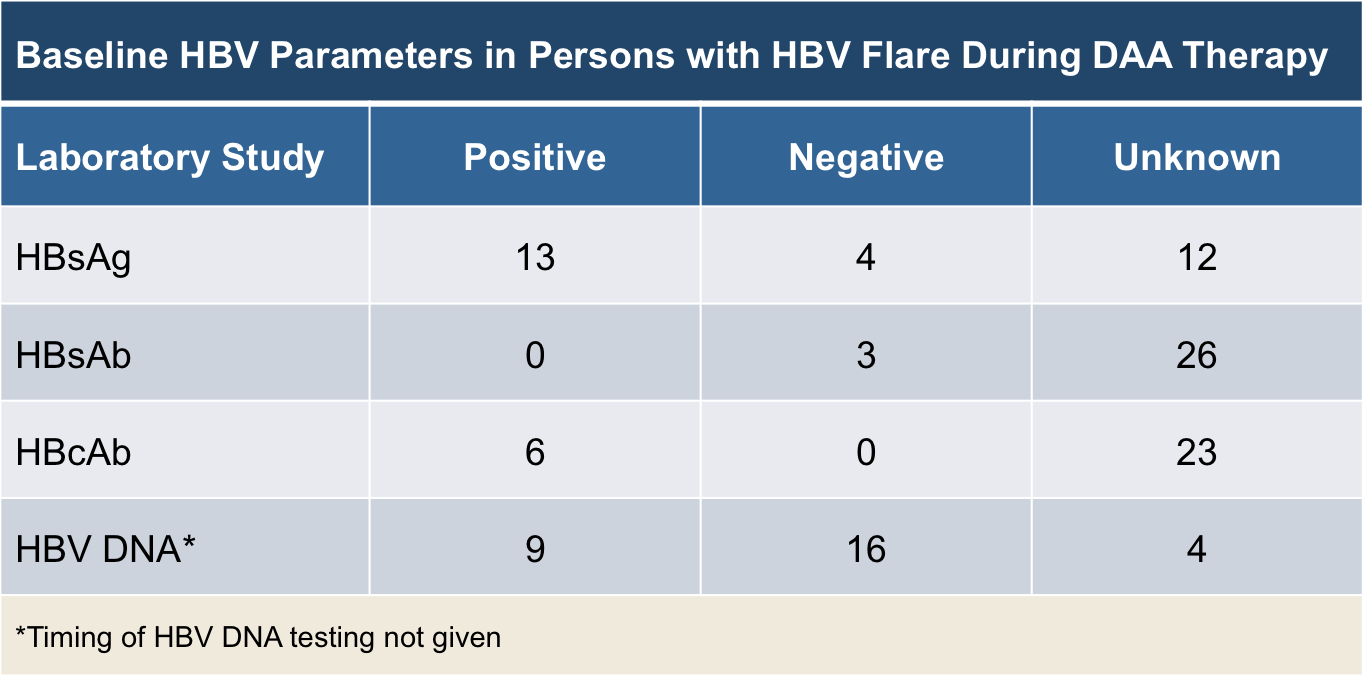
Core Concepts Monitoring During And After Hcv Treatment Treatment Of Chronic Hepatitis C Infection Hepatitis C Online

Performance Evaluation Of The Versant Hcv Rna Qualitative Assay By Using Transcription Mediated Amplification Journal Of Clinical Microbiology
What Is Hepatitis C Viral Load
Q Tbn 3aand9gcsf2 Pox2upnotm56whrd0khgrimaadlykabtxj Pdazc607ytg Usqp Cau
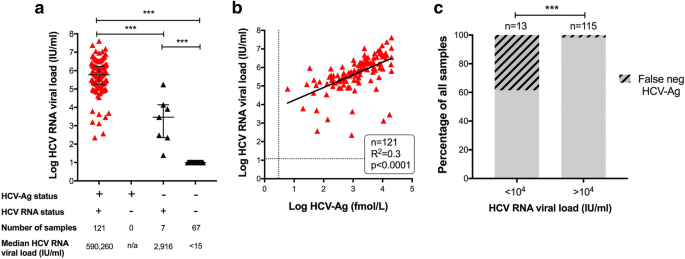
Hepatitis Virus Hcv Diagnosis And Access To Treatment In A Uk Cohort Bmc Infectious Diseases Full Text

Commercially Available Quantitative Real Time Pcr Based Hepatitis C Download Table
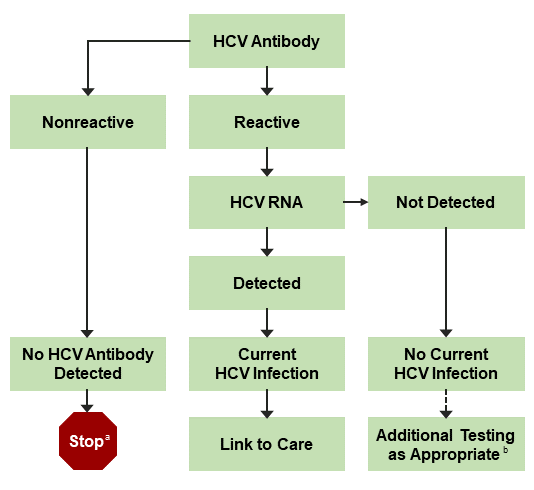
Hcv Testing And Linkage To Care Hcv Guidance

Qualitative And Quantitative Results Of Hepatitis C Virus Hcv Antigen Download Table
Hepatitis C Antibody Test Results And What To Expect



