Hcv Rna Quantitative Real Time Pcr Labcorp

Labmedica International January By Globetech Issuu
Cdos Halfpenny Com Labcorp Chifranciscan Report Page 1
2
Q Tbn 3aand9gcsz 8w2 Fellupqayhpcbslipgwwihndulzaigwj062n Wtwybu Usqp Cau
2

Std Testing Order An Online Std Blood Test For Hiv Herpes Syphilis Walk In Lab
Retesting for HCV RNA in a subsequent blood sample, at least 6 months after the initial RNA test, is recommended to confirm chronic infection HCV RNA test Real-time PCR:.
Hcv rna quantitative real time pcr labcorp. Clinical Significance HIV-1 RNA, Quantitative, Real-Time PCR - This test is intended for use in conjunction with clinical presentation and other laboratory markers of disease progress for the clinical management of HIV-1 infected patients. The HCV RNA PCR test is conducted through a process called polymerase chain reaction (PCR). HIV-1 Quantitative, Real-Time PCR (Roche COBAS ® TaqMan ® 2.0) V1000:.
Anyone who has ever injected illegal drugs, people with HIV, anyone who received a blood transfusion or organ transplant prior to July 1992, children born to HCV-infected. Thermodynamically modulated partially double-stranded linear DNA probe design for homogeneous real-time PCR. See Hepatitis C Virus (HCV) Genotyping, Nonreflex for more information.
The response to treatment is considered good when the quantitative HCV RNA measurement drops and the virus eventually becomes completely undetectable. LABCORP CODE LABCORP DIAGNOSTIC NAME Hepatitis C (HCV) Antibody with Reflex to HCV, RNA, Qualitative, Real-Time PCR Hepatitis C (HCV) Antibody with Reflex to HCV, RNA, Quantitative, Real-Time PCR DIAGNOSTIC NAME Hepatitis C Viral (HCV) RNA, Quantitative, Real-Time PCR (viral load) DESCRIPTION Reflex testing is the preferred. Real -Time Reverse PCR (RT PCR References:.
Hepatitis C Virus (HCV) by Quantitative NAAT:. During each cycle the fluorescence is measured, which greatly increases the dynamic range of the reaction, because the amount of fluorescence is proportional to the amount of PCR product. Hepatitis C Virus (HCV) Genotyping With 1a Subtype Reflex to Hepatitis C Virus (HCV) NS5A Drug Resistance Assay.
Hepatitis C virus RNA quantification results obtained in 18 laboratories using real-time PCR methods with 10 negative samples and 22 sample dilutions (viral loads of 0.5 to 500 IU/ml) showed a score of correct results of up to 93.5%. Real-time PCR and RT-PCR (also known as quantitative or qPCR) allows the quantification of exact quantity of DNA, cDNA, and RNA targets to starting quantity. Hepatitis C Virus (HCV) Antibody With Reflex to Quantitative Real-time PCR HCV screening recommendations (AASLD, IDSA, ACG) include:.
1), Version 2 (CAP/CTM Ver. Used to obtain HCV viral load, HCV genotype and NS5a resistance testing when considering treatment with ZepatierTM or in other circumstances where all three tests are warranted;. Quantitative Real-Time PCR 8472(X) Hepatitis C Virus (HCV) Antibody With Reflex for HCV Antibody Verification () Liver Fibrosis, FibroTest-ActiTest Panel 926(X) Hepatitis C Virus (HCV) FibroSURE® () Hepatitis C Viral RNA Genotype, LiPA (X) Hepatitis C Virus (HCV) Genotyping () Hepatitis C Viral RNA, Quantitative, Real.
Detection of HCV RNA indicates that the virus is replicating and therefore is evidence of active infection. Background and Aims Monitoring of chronic Hepatitis C (CHC) treatment relies on HCV RNA quantification by means of real-time PCR methods. Abbott m00® real-time HCV assay References Huang S, Salituro J, Tang N, et al.
Hepatitis C Virus Antibody by CIA:. 0.79 IV or less:. Hepatitis C Viral RNA, Quantitative Real-time PCR with Reflex to Genotype, LiPA ® (X) Confirms active infection and establishes baseline viral load.
This test is used to determine the number of international units. If you are unsure or if you just want to screen for this virus, you may want to order the Hepatitis C Virus Antibody test. Quantitative measurement of HIV-1 RNA in plasma.
Hepatitis C Antibody by CIA Index:. - “Hepatitis C Virus (HCV) Antibody With Reflex to Quantitative Real-time PCR” and - “Hepatitis C Virus (HCV) Antibody With Reflex to Qualitative NAA”. Hepatitis C is an infectious disease affecting the liver, caused by the hepatitis C virus (HCV).
If shipment will be delayed for >24 hours, freeze serum at -70° C until shipment on dry ice. For those who are HCV-positive, this test will determine the number of IU of the hepatitis C virus' RNA per milliliter of serum or plasma. Virus (HCV), Quantitative, Real-time PCR (Nongraphical) to determine the number of international units (IU) of hepatitis C virus (HCV) RNA per milliliter of serum or plasma in known HCV-positive patients.
If Hepatitis C Antibody is reactive, then Hepatitis C Viral RNA, Quantitative, Real-Time PCR will be performed at an additional charge. The HCV RNA PCR test is a blood test. They use a process called a polymerase chain reaction (PCR).
Utilizing a real-time assay with high sensitivity and broad dynamic range, the variability of serial HCV RNA that we observed was consistent with earlier findings (2, 8, 10, 12, 17), with no clinically significant differences between values from an initial reflex and subsequent direct sample and from two direct samples (Table 1;. If the assay DID NOT DETECT the virus, the test result will be reported as "Not Detected" If the assay DETECTED the presence of the virus but was not able to accurately quantify the number of copies, the test result will be reported as "< 10 Detected". LabCorp Test Number Clinical Use;.
Two standardized HCV RNA quantitative assays based on real-time PCR were recently developed (Abbott RealTime HCV and Roche Cobas TaqMan HCV). Performance characteristics of a quantitative, homogeneous TaqMan RT-PCR test for HCV RNA. However, these data also show substantial remaining variability.
A lab technician looks for the genetic material of the HCV virus, or its ribonucleic acid (RNA). LabCorp test details for Hepatitis C Virus (HCV) Antibody With Reflex to Quantitative Real-time PCR. The data presented here suggest that similar improvements in quantitative precision can be achieved for EBV PCR using real-time methods.
HEPATITIS C VIRAL RNA (HCV RNA) QUANTITATIVE REAL TIME PCR 4500 This test is intended for use as an aid in management of HCV infected patients and is not intended for use in the initial diagnosis or confirmation of HCV infection. As a result, clinicians who have used the bDNA tests for longitudinal studies of their patient’s viral load may want to re-establish a new baseline viral load using the real-time PCR viral load assay we have recommended as the alternative (test code ). This assay requires an HCV genotype of either 1a or 1b.
2) and the Abbott RealTime HCV (ART. Patients should have a quantitative HCV RNA level obtained at baseline prior to starting therapy and 12 weeks after completion of treatment. Hepatitis C Viral RNA, Quantitative, Real-Time PCR (X) Utilized to monitor response to therapy;.
1.00 to 10.99 IV:. Quantitative HCV RNA testing can be considered at the end of therapy and at 24 weeks or later after completion of antiviral therapy. These tests are approved for diagnosis of infection.
The result is reported as either "detected" or "not detected." Explanation of test results:. LabCorp offers the following NAT tests:. Genotype used to guide treatment selection and duration;.
Order HCVQN / Hepatitis C Virus (HCV) RNA Detection and Quantification by Real-Time Reverse Transcription-PCR (RT-PCR). The HCV real-time PCR assay has a quantitative range of 15 IU/mL to 100,000,000 IU/mL. Quantitative HCV RNA is the preferred test for monitoring response to therapy, ideally with a lower limit of quantification in the range of 12 to 25 IU/mL.
Clinical Significance Hepatitis C Viral RNA, Quantitative, Real-Time PCR with Reflex to Genotype LiPA ® - Useful in monitoring response to therapy and/or disease progression. Reportable range is 15 to 100,000,000 IU/mL (1.18-8.00 log IU/mL). Infection with HCV results in a chronic infection in 50 to 80% of cases.
The quantitative HCV RNA test is used to monitor a patient who is currently on treatment. The generation of internationally agreed upon calibration standards would be a first step toward such a goal. Hepatitis C Virus (HCV) is a major cause of hepatitis.The clinical symptoms of an HCV infection are variable.
There are two approaches to this process:. If the viral load is just at or above this LOD, but less than 15 IU/mL, the assay can determine that HCV RNA is present but cannot provide a reliable quantitative result. Performance characteristics of a quantitative, homogeneous TaqMan™ RT-PCR test for HCV RNA.
This test quantifies HCV RNA of free HCV virions in serum / plasma. Clinical Significance Hepatitis C Viral RNA, Quantitative, Real-Time PCR - Useful in monitoring therapy and/or disease progression. This is a quantitative real-time PCR assay with a lower limit of quantification (LOQ) of 15 IU/mL;.
This test is typically only done for known HCV positive individuals. These assays support new USPSTF screening. 0.80 to 0.99 IV:.
If a qualitative RNA test is positive (detected), then it is confirmed that the patient has chronic hepatitis C. The limit of detection (LOD) is slightly lower, at 10 IU/mL to 13 IU/mL. The clinical symptoms of an HCV infection are variable.
The HCV real-time PCR assay has a quantitative range of 15 IU/mL to 100,000,000 IU/mL. Both have a high sensitivity (based on the lower limit for detecting HCV RNA) and a great dynamic range and were standardized using the second WHO panel. Real-time Reverse Transcription-Polymerase Chain Reaction, Quantitative.
HCV RNA level of below 25 IU/mL in serum or plasma at 12 weeks after ending therapy is the therapeutic goal and indicates an SVR is achieved. If the HCV Quantitative PCR result is greater than or equal to 179 IU/mL (2.3 log IU/mL), the HCV Genotype by PCR and Line Probe assay will be added. On April 2, 12 we will no longer offer the HCV bDNA tests.
Specimens that are repeatedly reactive by screening tests should be confirmed by a more HCV-specific test. Methods Comparative analysis between three commercial assays (Roche COBAS AmpliPrep/COBAS TaqMan Version 1 (CAP/CTM Ver. Reportable range is 15 to 100,000,000 IU/mL (1.18-8.00 log IU/mL).
If HCV RNA, PCR QT is ≥300 IU/mL. 11.00 IV or greater:. The Quantitative, real-time PCR test measures the IU (international units) of the HCV (hepatitis C) RNA per millimeter of plasma or serum.
AccuPower® HCV Quantitative RT-PCR Kit is an in vitro diagnostic kit designed for the quantification of hepatitis C virus (HCV) RNA in human samples such as serum and EDTA-plasma through real-time PCR. The qualitative HCV RNA tests will report whether the hepatitis C virus is present in the bloodstream or not. Mayo Medical Laboratories Web Page January 18.
A result of ">100,000,000 IU/mL (>8.00 log IU/mL)" indicates the presence of active HCV viral replication, and the HCV RNA level present cannot be quantified accurately above this upper limit of. HEPATITIS C (HCV) RNA QUANTITATIVE PCR General Information Lab Order Codes:. 2 LabCorp also offers a convenient HCV cascade test - Hepatitis C Virus (HCV) Antibody Cascade to Quantitative PCR and Genotyping that reflexes a positive antibody test to quantitative PCR and then to genotyping when the viral load.
References Kleiber J, Walter T, Haberhausen G, Tsang S, Babiel R, Rosenstraus M. Using HCV real‐time PCR as a supplementary test, plus references from the antibody S/Co ratio, we were able to address confirmation of the positive antibody screening results and provide useful information generally required by clinicians, including the needs of further laboratory testing or clinical follow‐up, as well as HCV viral titers. Monitoring HCV RNA levels over time is important to assess disease progression and /or monitoring a.
Hepatitis C Virus (HCV) RNA Detection and Quantification by Real-Time Reverse Transcription-PCR (RT-PCR), Serum Hepatitis C Virus (HCV) RNA Quantification with Reflex to HCV Genotype, Serum Hepatitis C Virus Antibody Confirmation, Serum. Assay specific analytical sensitivities may impact therapy management. Kleiber J, Walter T, Haberhausen G, Tsang S, Babiel R, Rosenstraus M.
A qualitative RNA test (to detect the presence or absence of HCV RNA) or a quantitative RNA test (to detect the amount of RNA) is recommended1,2. Clinical Significance Hepatitis C Antibody with Reflex to HCV, RNA, Quantitative, Real-Time PCR - Hepatitis C Virus (HCV) is a major cause of hepatitis. For detection and quantification of hepatitis C virus (HCV) RNA in human serum or plasma, and to monitor disease progression in chronic HCV infection and response to anti-HCV therapy.
Monitoring HCV RNA levels over time is important to assess disease progression and/or monitoring a patient's response to anti-HCV therapy. This assay requires an HCV genotype of either 1a or 1b. Hepatitis C Viral RNA Quantitative Real-Time PCR w/Re˜exes 3 mL frozen EDTA plasma from a lavender-top tube (1.8 mL minimum).
LabCorp offers test – “Hepatitis C Virus (HCV) Antibody with Reflex to Quantitative Real-Time PCR” using FDA-approved antibody and NAT tests to aid in the screening and follow up of.

Pdf Clinical Laboratory Role In Viral Pandemic Response Focus On Covid 19
Http Www State Wv Us Admin Purchase Bids Fy12 B Bhs113 02 Pdf

Order Std Test Online From 79 Visit Lab Or Test At Home
Www Labcorp Com Tests Related Documents L9609
Www Journalofsubstanceabusetreatment Com Article S0740 5472 17 2 Pdf
Http Www Acgov Org Board Bos Calendar Documents Docsagendareg 06 05 12 Health care services Regular calendar Hcsa Unilab Corporation Pdf
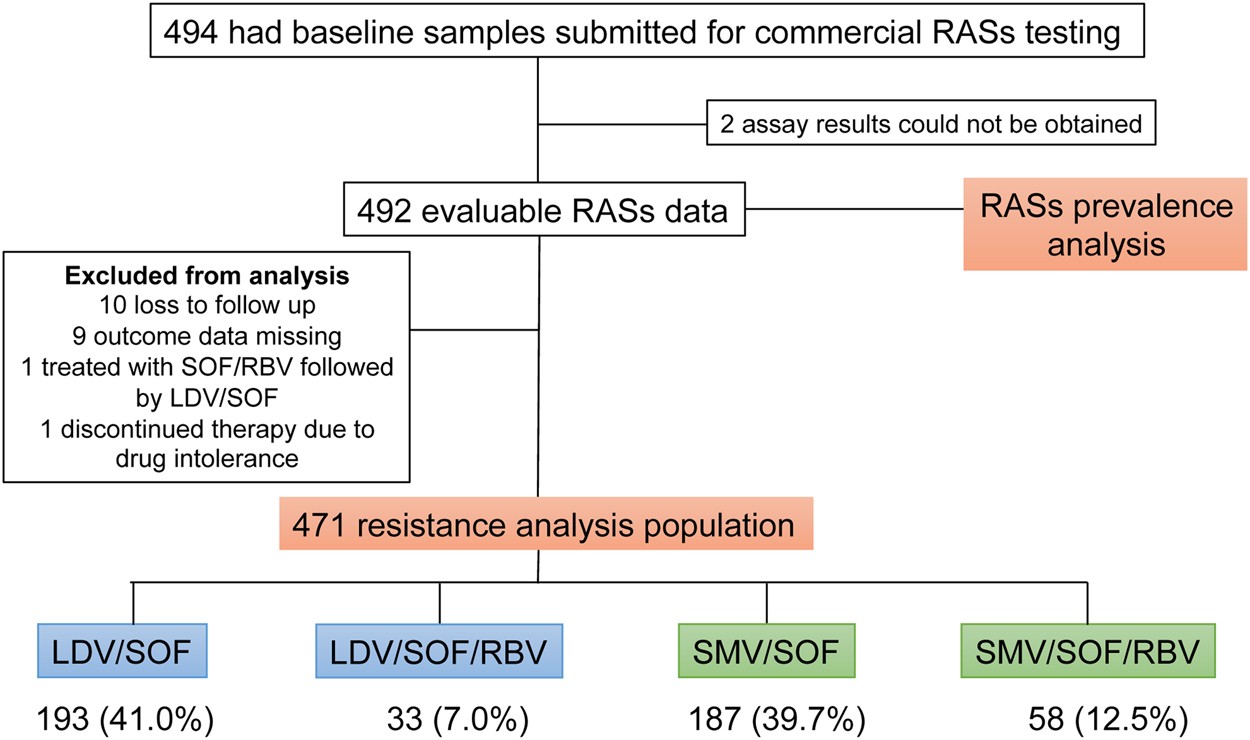
Prevalence And Impact Of Baseline Resistance Associated Substitutions On The Efficacy Of Ledipasvir Sofosbuvir Or Simeprevir Sofosbuvir Against Gt1 Hcv Infection Scientific Reports

Multicenter Evaluation Of The New Abbott Realtime Assays For Quantitative Detection Of Human Immunodeficiency Virus Type 1 And Hepatitis C Virus Rna Journal Of Clinical Microbiology

Jrp Innovative Approach For Enhancing Testing Of Hiv Hepatitis B And Hepatitis C In The General Population Protocol For An Acceptability And Feasibility Study Barotest 16 Lydie Jmir Research Protocols
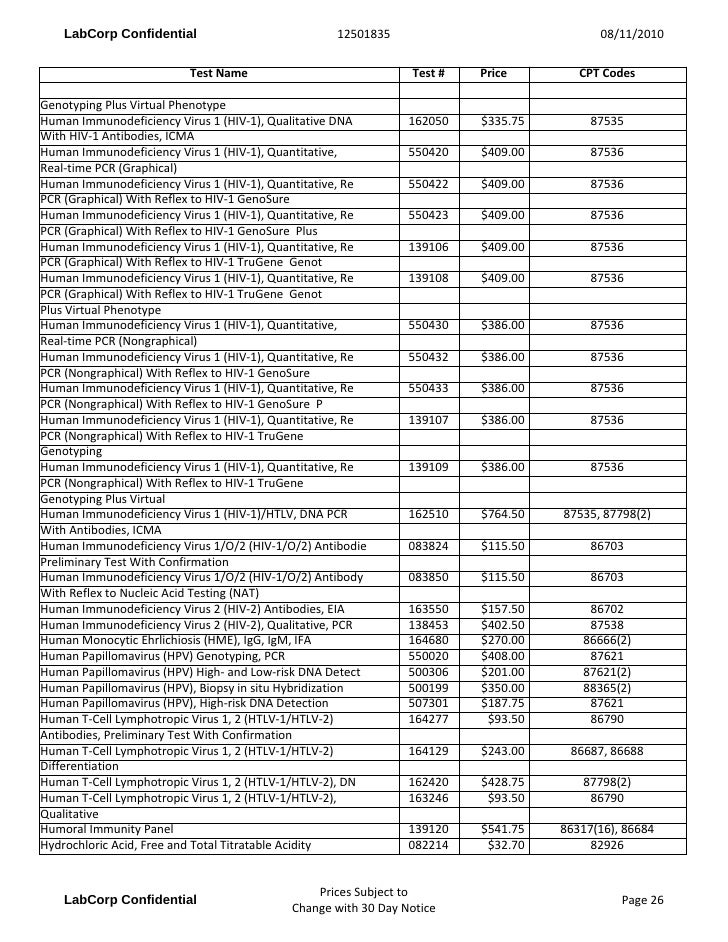
Lab Testing Pricing
2
2
2

L Sign Cd 9l Is A Liver Specific Capture Receptor For Hepatitis C Virus Pnas
Http Www Acgov Org Board Bos Calendar Documents Docsagendareg 06 05 12 Health care services Regular calendar Hcsa Unilab Corporation Pdf
Std Testing Order An Online Std Blood Test For Hiv Herpes Syphilis Walk In Lab
Www Agnesian Com Sites Default Files Forms 06 08 Pdf

Order Std Test Online From 79 Visit Lab Or Test At Home
Www Labcorp Com Tests Related Documents L9609

Hepatitis C Virus Core Antigen A Potential Alternative To Hcv Rna Testing Among Persons With Substance Use Disorders Sciencedirect
Www Labcorp Com Tests Related Documents L9609

Comparison Of Qualitative Cobas Amplicor Hcv 2 0 Versus Versant Hcv Rna And Quantitative Cobas Amplicor Hcv Monitor 2 0 Versus Versant Hcv Rna 3 0 Assays For Hepatitis C Virus Hcv Rna Detection And
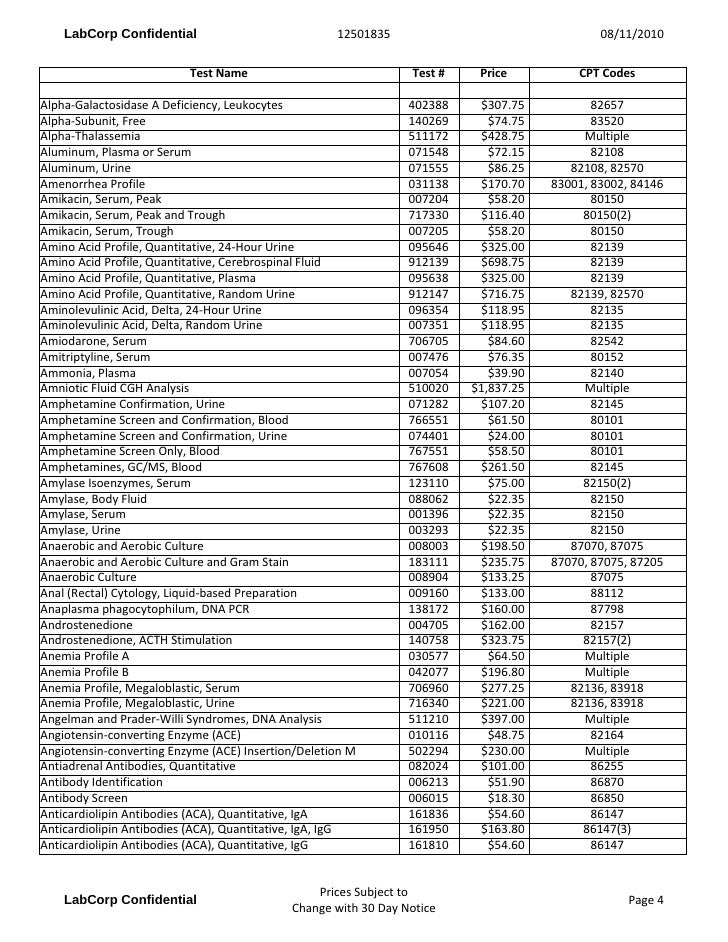
Lab Testing Pricing
Ir Labcorp Com Static Files 091eb7a1 033c 4a3c A0d8 Ac5fbe
2
Www Testmenu Com Healthnetworklaboratories Testdirectory Sitefile Filename Sidebar 5clabhandbookhnl613 Pdf
Cdos Halfpenny Com Labcorp Slh Report Page 1

Importance Of Patient Education And Monitoring Among Hcv Infected Patients Selected For Anti Viral Treatment Wentworth Kotara 12 Clinical Liver Disease Wiley Online Library
2
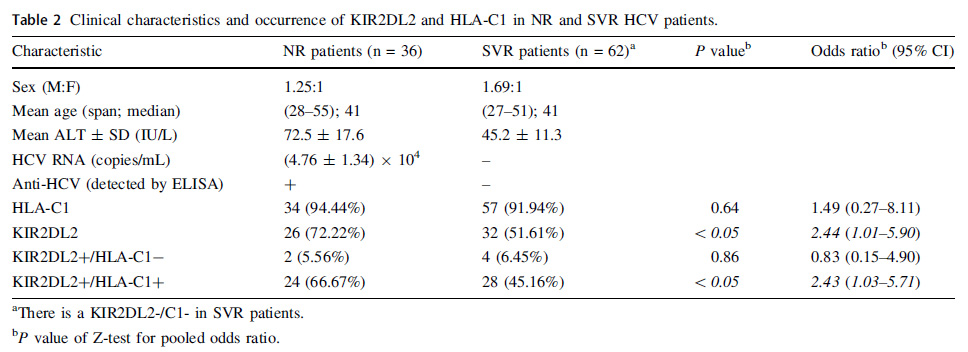
Kir2dl2 C1 Is A Risk Factor For Chronic Infection And Associated With Non Response To Peg Ifn And Rbv Combination Therapy In Hepatitis C Virus Genotype 1b Patients In China
Academic Oup Com Labmed Article Pdf 38 2 85 Labmed38 0085 Pdf
2
Http Www State Wv Us Admin Purchase Bids Fy12 B Bhs113 02 Pdf
Q Tbn 3aand9gcsrtopdmolybcrgnsfq33uxtkfevfhusdhq0tmkog6tir7dhxtl Usqp Cau

An Overview Of Hiv And Hcv Viral Load Monitoring Medical Laboratory Observer
2
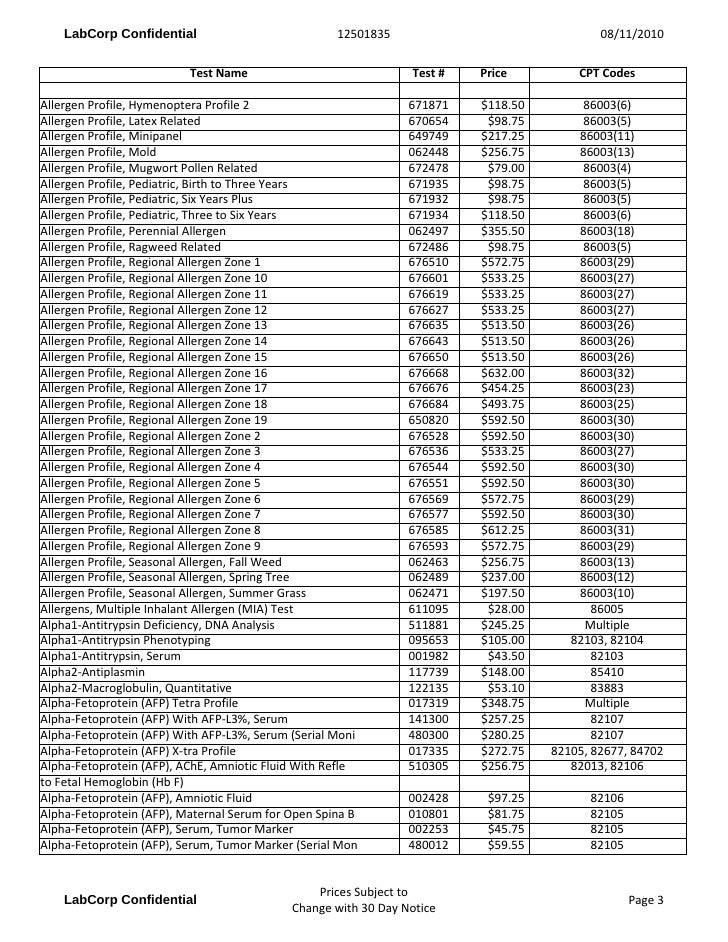
Lab Testing Pricing
sldpubs Onlinelibrary Wiley Com Doi Pdf 10 1002 Hep
Http Www Doh Wa Gov Portals 1 Documents 56 Hcvtestingguideforproviders Pdf
Http Www Acgov Org Board Bos Calendar Documents Docsagendareg 06 05 12 Health care services Regular calendar Hcsa Unilab Corporation Pdf
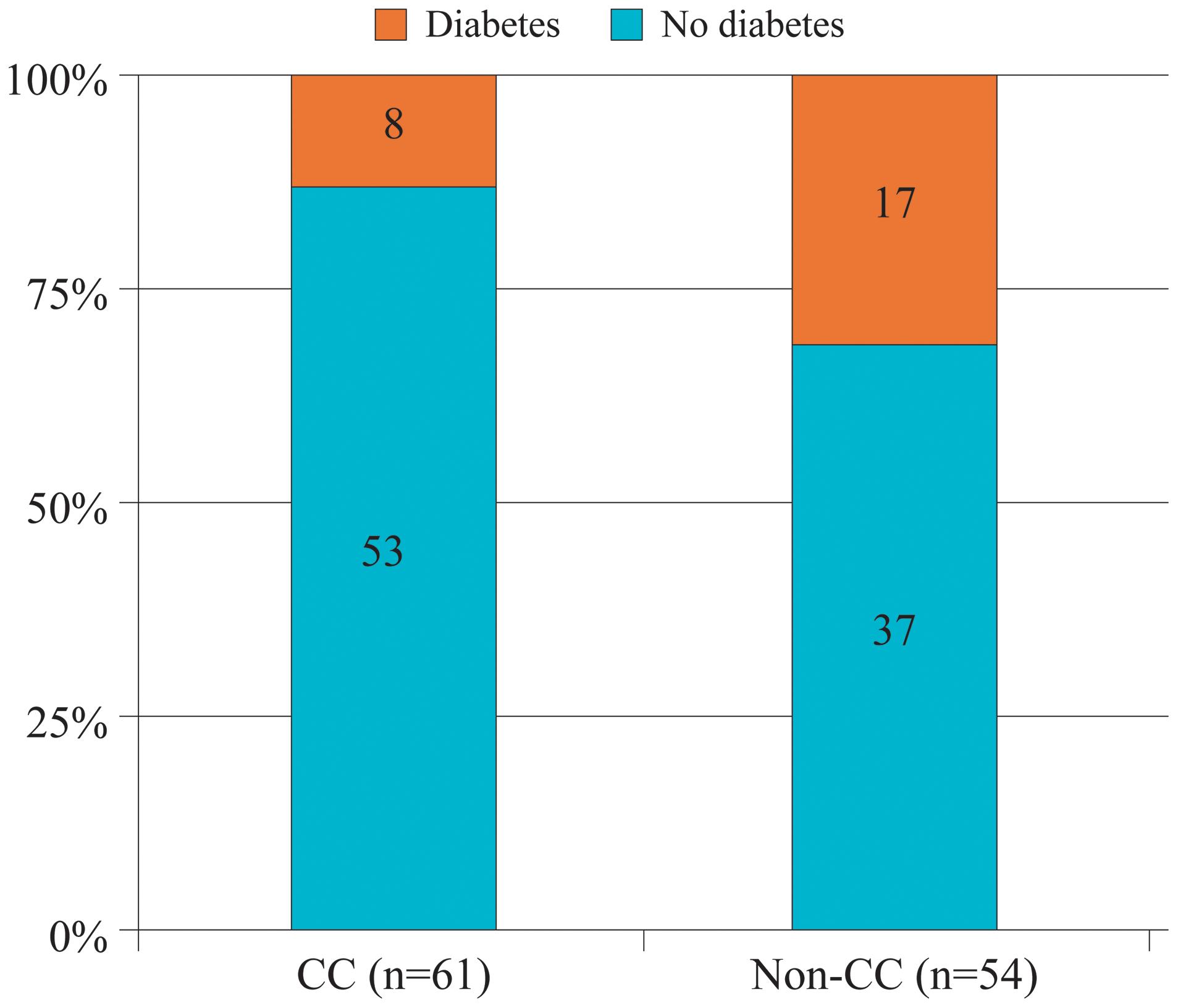
Association Of Overt Diabetes Mellitus With The Non Cc But Not The Cc Genotype Of Interleukin 28b In Hepatitis C Virus Infected Patients
Www Labcorp Com Assets Media 2332
Www Nrl Ae Public Uploads Downloads Gastrotest List Pdf
Www Labcorp Com Tests Related Documents L9609
Www Gsaadvantage Gov Ref Text V797p7041a V797p7041a Online Htm

L Sign Cd 9l Is A Liver Specific Capture Receptor For Hepatitis C Virus Pnas
2
Http Procure Ohio Gov Pdf Labcorp price list dated Pdf
Www Hcv Com Content Dam Hcv Pdf Pre Treatment Guide Pdf

Order Std Test Online From 79 Visit Lab Or Test At Home

Factors Influencing Treatment Outcome In Hepatitis C Virus Minority Patients At An Inner City Hospital A Strobe Complaint Article Abstract Europe Pmc
Files Labcorp Com Labcorp D8 06 226 04 wh may Email Pdf Pdf

Order Std Test Online From 79 Visit Lab Or Test At Home
Q Tbn 3aand9gcqjshgdkuojadkpeygb9armztprqpt4ehd 2faue E5njovzts3 Usqp Cau

Order Std Test Online From 79 Visit Lab Or Test At Home

Hcv Compliance And Treatment Success Rates Are Higher With Daas In Structured Hcv Clinics Compared To General Hepatology Clinics Abstract Europe Pmc
Http Www Dynacaremilwaukee Com Downloads Service Directory 10 Pdf

Bmlhtfw55n Xqm

Order Std Test Online From 79 Visit Lab Or Test At Home

Order Std Test Online From 79 Visit Lab Or Test At Home
Www Labcorp Com Tests Related Documents L9609

Hepatitis C Challenges And Opportunities In The Laboratory Diagnosis Of Infection Medical Laboratory Observer

Sampleshop Biomex Gmbh
Ir Labcorp Com Static Files 091eb7a1 033c 4a3c A0d8 Ac5fbe
Http Www Hepcap Org Wp Content Uploads 17 10 Final Hcv Testing Guide Pdf Online Download File And Printable Pdf
Http Www Dynacaremilwaukee Com Downloads Service Directory 10 Pdf
Www Labcorp Com Tests Related Documents L
Www Gsaadvantage Gov Ref Text V797p7041a V797p7041a Online Htm

Hepatitis C Virus Core Antigen A Potential Alternative To Hcv Rna Testing Among Persons With Substance Use Disorders Sciencedirect
2

Jrp Innovative Approach For Enhancing Testing Of Hiv Hepatitis B And Hepatitis C In The General Population Protocol For An Acceptability And Feasibility Study Barotest 16 Lydie Jmir Research Protocols
Q Tbn 3aand9gcqkxzpmzifipt86zu4 M X8toverctgru69h94kyrhe 2xsnw5j Usqp Cau
Academic Oup Com Labmed Article Pdf 38 2 85 Labmed38 0085 Pdf

Multicenter Evaluation Of The New Abbott Realtime Assays For Quantitative Detection Of Human Immunodeficiency Virus Type 1 And Hepatitis C Virus Rna Journal Of Clinical Microbiology

Order Std Test Online From 79 Visit Lab Or Test At Home

Pdf Multicenter Comparison Of Different Real Time Pcr Assays For Quantitative Detection Of Epstein Barr Virus
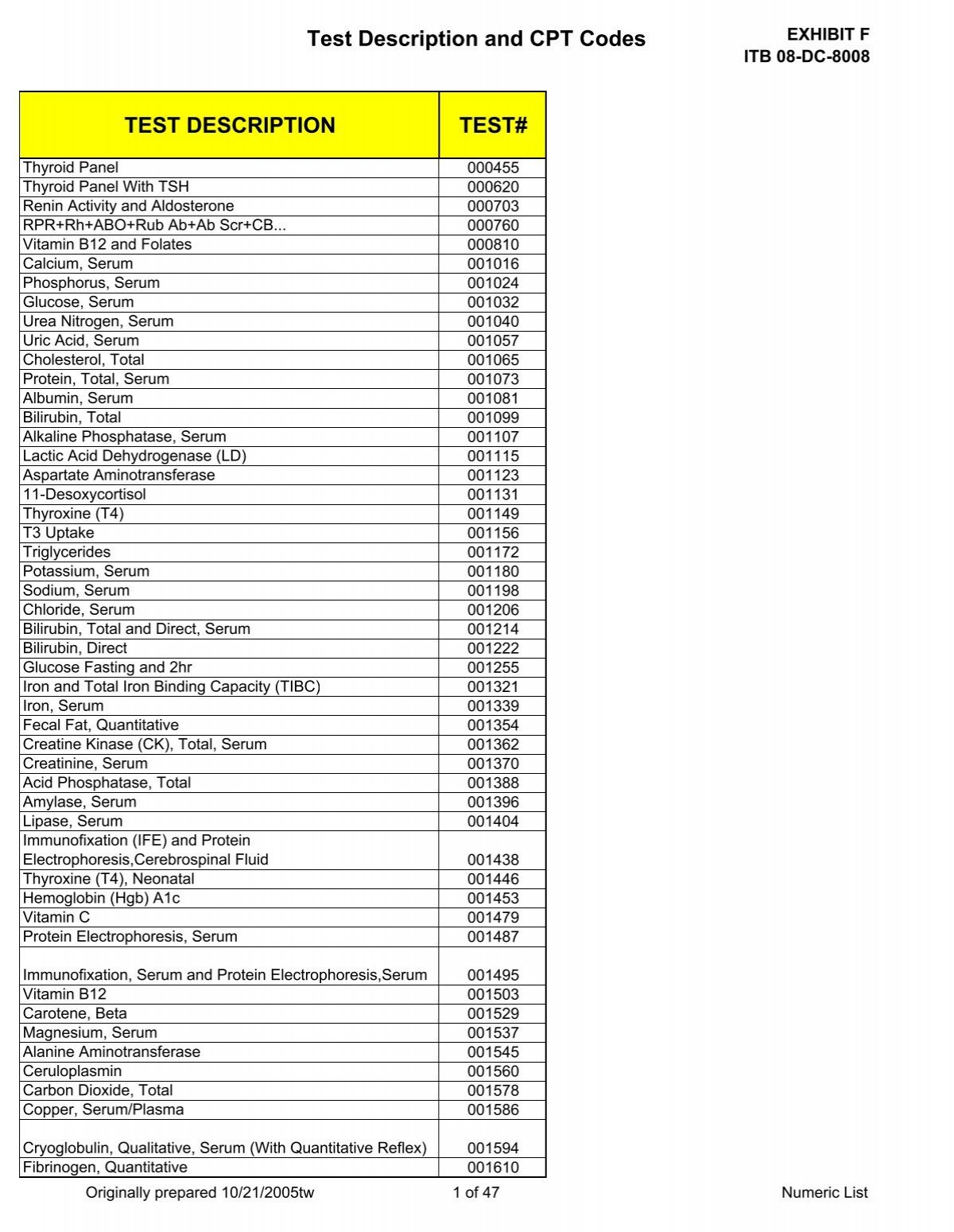
Test Description And Cpt Codes
Files Labcorp Com Labcorp D8 02 L 0417 2 Pdf

Order Std Test Online From 79 Visit Lab Or Test At Home
Files Labcorp Com Labcorp D8 06 226 04 wh may Email Pdf Pdf
Http Www State Wv Us Admin Purchase Bids Fy12 B Bhs113 02 Pdf
Www Gsaadvantage Gov Ref Text V797p7041a V797p7041a Online Htm
Www Labcorp Com Assets Media 2332
Files Labcorp Com Labcorp D8 06 226 04 wh may Email Pdf Pdf
Www Journalofsubstanceabusetreatment Com Article S0740 5472 17 2 Pdf
2
Hiv 1 Rna By Pcr Labcorp Find Lab Tests Online
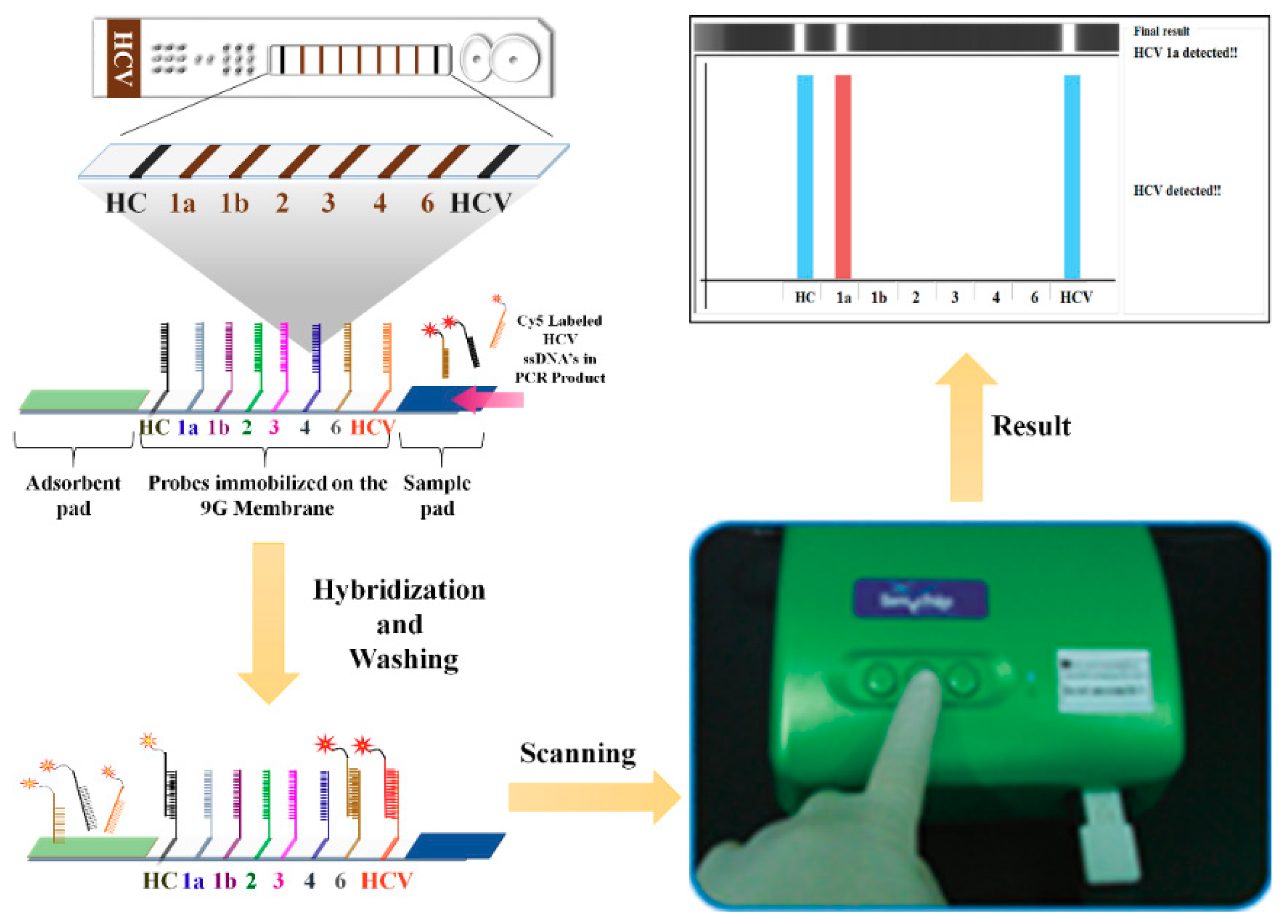
Sensors Free Full Text Hcv Detection Discrimination And Genotyping Technologies
2

Woa1 Oligonucleotides For The Detection Of Hepatitis B Virus Google Patents
Www Hcv Com Content Dam Hcv Pdf Pre Treatment Guide Pdf
2
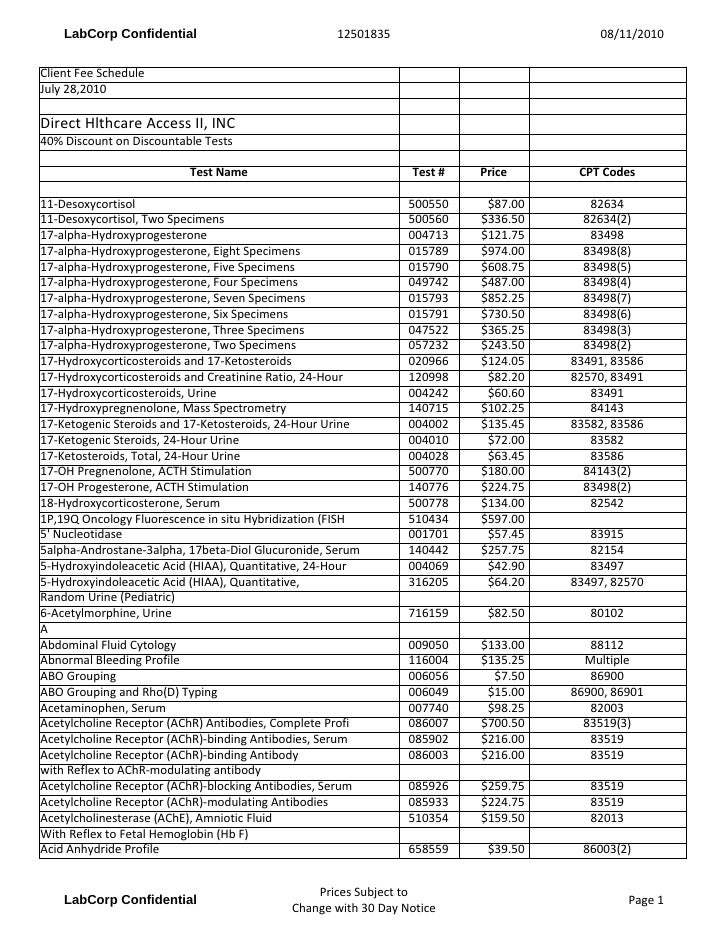
Lab Testing Pricing
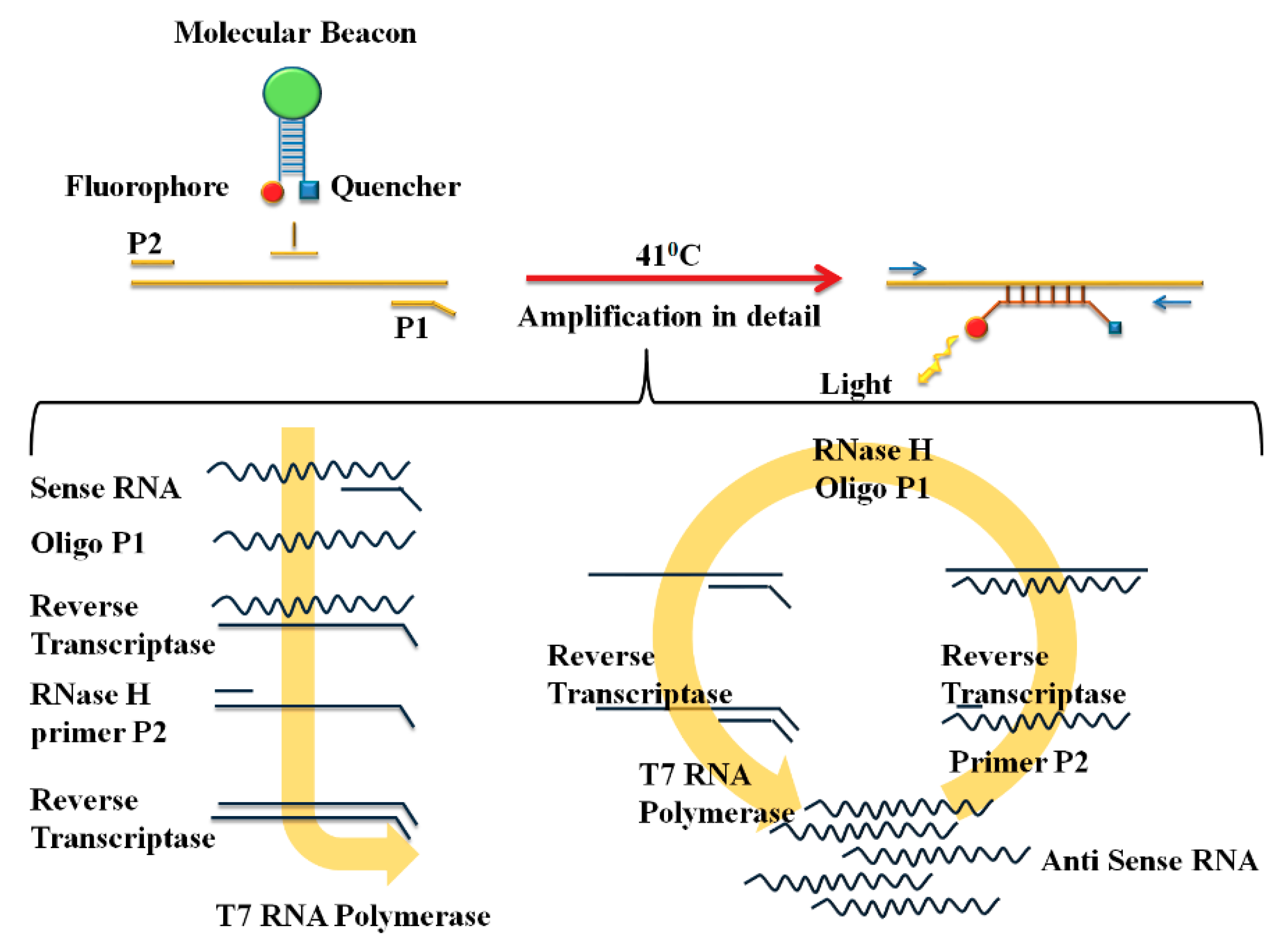
Sensors Free Full Text Hcv Detection Discrimination And Genotyping Technologies
2
Hcv Rna Pcr How This Hepatitis C Virus Test Works Results More
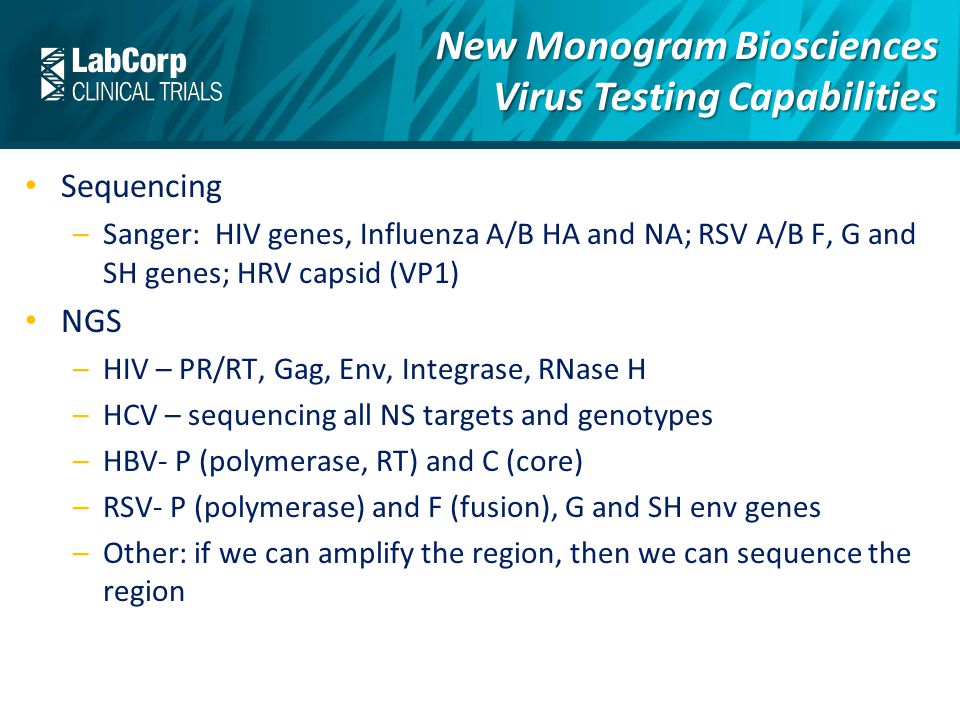
New Monogram Biosciences Testing Capabilities Influenza Rsv Ebola And Other Respiratory And Enveloped Viruses Ppt Video Online Download

Liver Function Blood Test Liver Blood Tests Walk In Lab



